Brain Tumor Res Treat.
2014 Apr;2(1):29-35. 10.14791/btrt.2014.2.1.29.
Aberrant CpG Islands Hypermethylation Profiles in Malignant Gliomas
- Affiliations
-
- 1Department of Neurosurgery, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea. drson@dsmc.or.kr
- KMID: 1734665
- DOI: http://doi.org/10.14791/btrt.2014.2.1.29
Abstract
- BACKGROUND
The authors analyzed whether the promoter hypermethylation of cancer-related genes was involved in the tumorigenesis of malignant gliomas.
METHODS
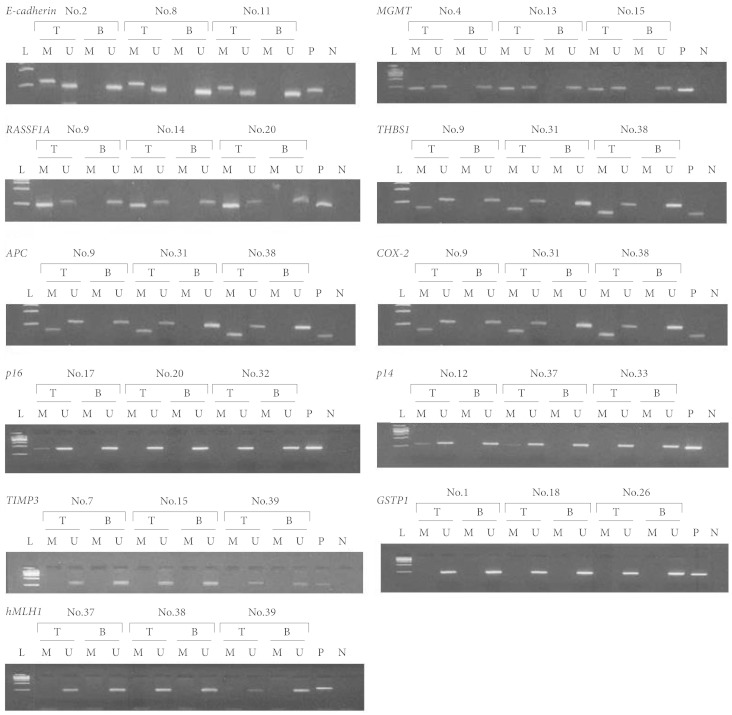
A total of 29 patients received surgery and histologically confirmed to have malignant gliomas from January 2000 to December 2006. The promoter methylation status of several genes, which were reported to be frequently methylated in malignant gliomas, was investigated using methylation-specific polymerase chain reaction.
RESULTS
All cases of malignant gliomas represented the promoter hypermethylation in at least 2 or more genes tested. Of 29 tumors, 28 (96.55%) showed concurrent hypermethylation of 3 or more genes. Ras association domain family member 1, epithelial cadherin, O-6 methyl guanine DNA methyltransferase, thrombospondin 1, p14 and adenomatous polyposis coli were frequently methylated in high grade gliomas including glioblastomas, anaplastic astrocytomas, and anaplastic oligodendrogliomas.
CONCLUSION
Aberrant hypermethylation profile was closely related with malignant gliomas suggesting that epigenetic change may play a role in the development of malignant gliomas. Two or three target genes may provide useful clues to the development of the useful prognostic as well as diagnostic assays for malignant gliomas.
Keyword
MeSH Terms
Figure
Reference
-
2. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002; 3:415–428. PMID: 12042769.
Article3. Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004; 22:4632–4642. PMID: 15542813.
Article4. Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985; 313:144–147. PMID: 2981413.
Article5. Liu Y, Pang JC, Dong S, Mao B, Poon WS, Ng HK. Aberrant CpG island hypermethylation profile is associated with atypical and anaplastic meningiomas. Hum Pathol. 2005; 36:416–425. PMID: 15892004.
Article6. Laffaire J, Everhard S, Idbaih A, et al. Methylation profiling identifies 2 groups of gliomas according to their tumorigenesis. Neuro Oncol. 2011; 13:84–98. PMID: 20926426.
Article7. Hegi ME, zur Hausen A, Rüedi D, Malin G, Kleihues P. Hemizygous or homozygous deletion of the chromosomal region containing the p16INK4a gene is associated with amplification of the EGF receptor gene in glioblastomas. Int J Cancer. 1997; 73:57–63. PMID: 9334810.8. Mashiyama S, Murakami Y, Yoshimoto T, Sekiya T, Hayashi K. Detection of p53 gene mutations in human brain tumors by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1991; 6:1313–1318. PMID: 1886708.
Article9. Zhang L, Wang M, Wang W, Mo J. Incidence and prognostic value of multiple gene promoter methylations in gliomas. J Neurooncol. 2014; 116:349–356. PMID: 24197988.
Article10. Feltus FA, Lee EK, Costello JF, Plass C, Vertino PM. Predicting aberrant CpG island methylation. Proc Natl Acad Sci U S A. 2003; 100:12253–12258. PMID: 14519846.
Article11. Shen L, Kondo Y, Guo Y, et al. Genome-wide profiling of DNA methylation reveals a class of normally methylated CpG island promoters. PLoS Genet. 2007; 3:2023–2036. PMID: 17967063.
Article12. Gonzalgo ML, Pavlovich CP, Lee SM, Nelson WG. Prostate cancer detection by GSTP1 methylation analysis of postbiopsy urine specimens. Clin Cancer Res. 2003; 9:2673–2677. PMID: 12855646.13. Zare M, Jazii FR, Alivand MR, Nasseri NK, Malekzadeh R, Yazdanbod M. Qualitative analysis of Adenomatous Polyposis Coli promoter: hypermethylation, engagement and effects on survival of patients with esophageal cancer in a high risk region of the world, a potential molecular marker. BMC Cancer. 2009; 9:24. PMID: 19149902.
Article14. Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994; 22:2990–2997. PMID: 8065911.15. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996; 93:9821–9826. PMID: 8790415.
Article16. Goldmit M, Bergman Y. Monoallelic gene expression: a repertoire of recurrent themes. Immunol Rev. 2004; 200:197–214. PMID: 15242406.
Article17. Kloosterhof NK, de Rooi JJ, Kros M, et al. Molecular subtypes of glioma identified by genome-wide methylation profiling. Genes Chromosomes Cancer. 2013; 52:665–674. PMID: 23629961.
Article18. Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986; 321:209–213. PMID: 2423876.
Article19. Takai D, Jones PA. Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc Natl Acad Sci U S A. 2002; 99:3740–3745. PMID: 11891299.
Article20. Gama-Sosa MA, Slagel VA, Trewyn RW, et al. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983; 11:6883–6894. PMID: 6314264.
Article21. Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer. 2000; 36:2294–2300. PMID: 11094302.22. Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999; 21:163–167. PMID: 9988266.23. Cecener G, Tunca B, Egeli U, et al. The promoter hypermethylation status of GATA6, MGMT, and FHIT in glioblastoma. Cell Mol Neurobiol. 2012; 32:237–244. PMID: 21928112.
Article24. Lorente A, Mueller W, Urdangarín E, et al. RASSF1A, BLU, NORE1A, PTEN and MGMT expression and promoter methylation in gliomas and glioma cell lines and evidence of deregulated expression of de novo DNMTs. Brain Pathol. 2009; 19:279–292. PMID: 18616639.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CpG Island Hypermethylation in Gastric Carcinoma and Its Premalignant Lesions
- CpG Islands Detector: a Window-based CpG Island Search Tool
- DNA Methylation Changes in Human Cancers
- Understanding of epigenetics and DNA methylation
- Helicobacter pylori Eradication Modulates Aberrant CpG Island Hypermethylation in Gastric Carcinogenesis