Lab Anim Res.
2013 Dec;29(4):196-203. 10.5625/lar.2013.29.4.196.
Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cells in scaffold
- Affiliations
-
- 1Stem Cell Neuroplasticity Research Group, Kyungpook National University, Daegu, Korea. jsbae@knu.ac.kr
- 2Department of Physiology, Cell and Matrix Research Institute, School of Medicine, Kyungpook National University, Daegu, Korea.
- 3Department of Orthopaedic Surgery, Kyungpook National University Hospital, Daegu, Korea.
- 4Ossgen, Gyeongbuk Technopark, Gyeongbuk, Korea.
- 5Department of Laboratory Medicine, Yonsei Cell Therapy Center, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Laboratory Animal Medicine, College of Veterinary Medicine, Kyungpook National University, Daegu, Korea.
- KMID: 1716214
- DOI: http://doi.org/10.5625/lar.2013.29.4.196
Abstract
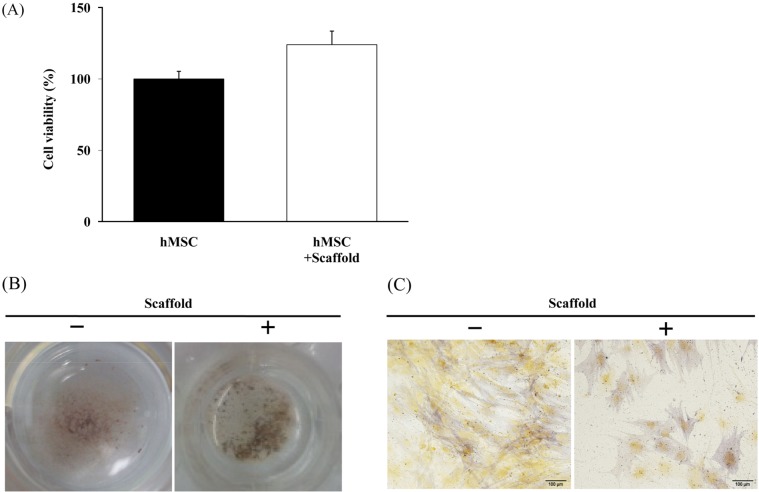
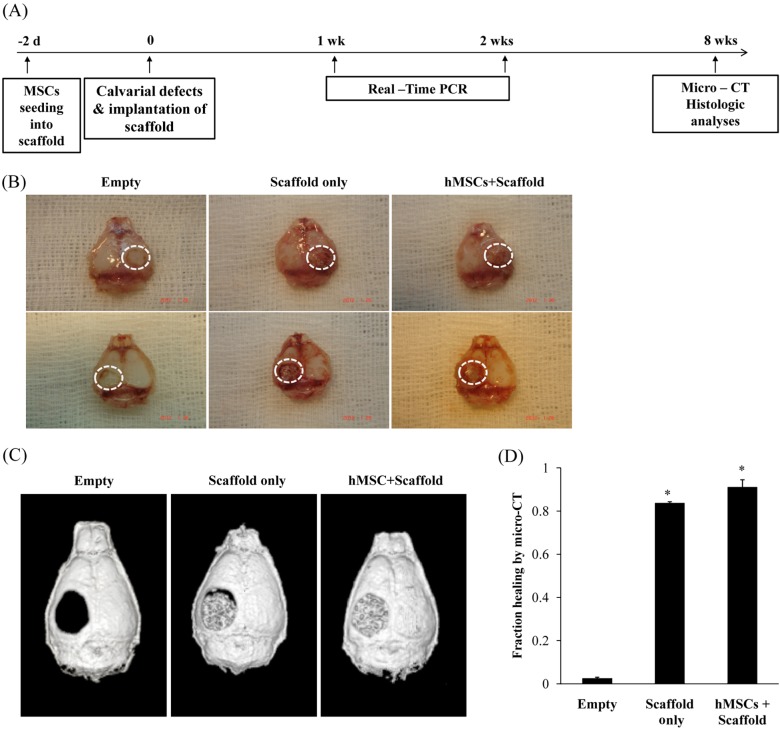
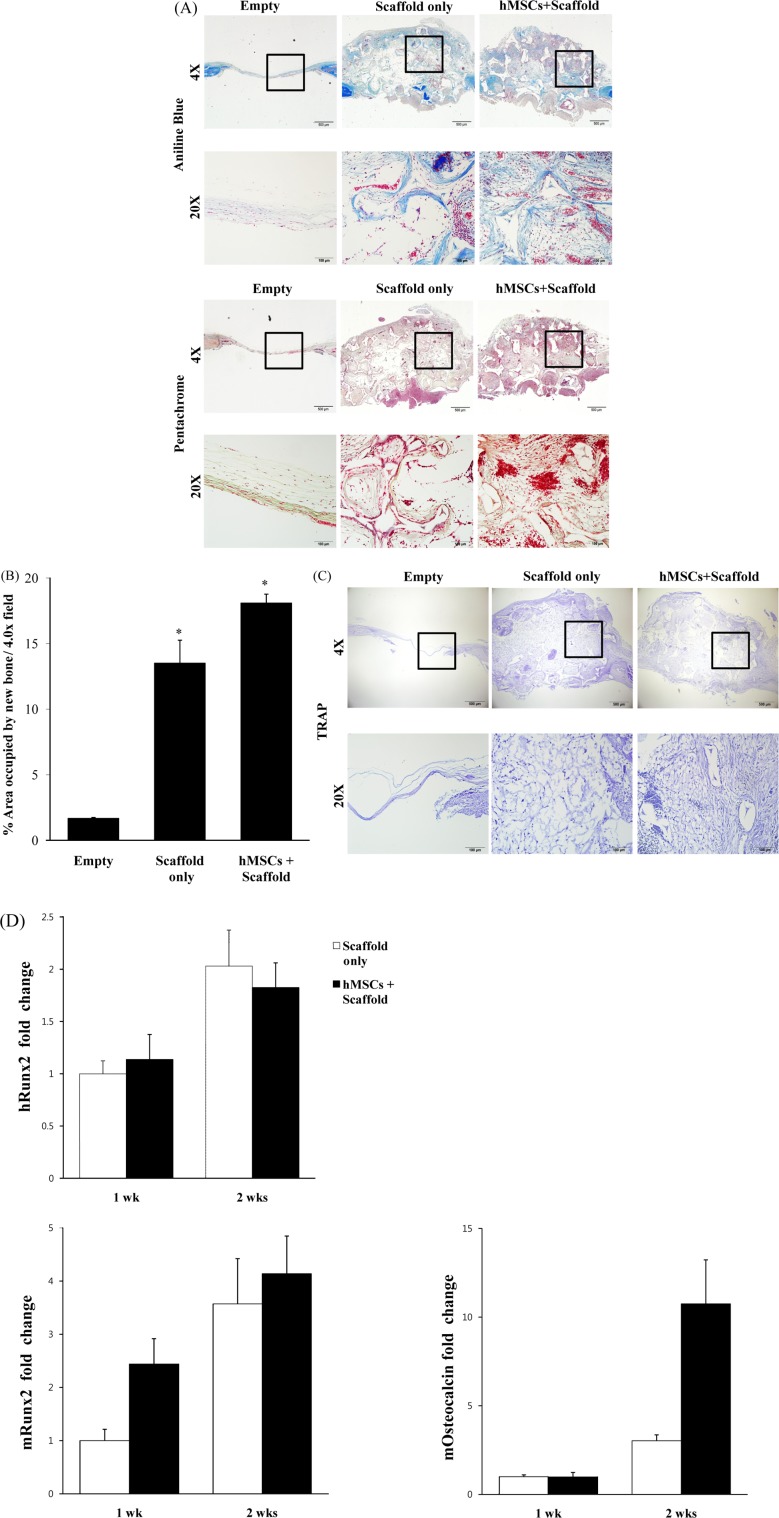
- Combination of tissue engineering and cell therapy represents a promising approach for bone regeneration. Human mesenchymal stem cells (hMSCs) have properties that include low immunogenicity, high proliferation rate, and multi-differentiation potential; therefore, they are an attractive seeding source for tissue engineering therapy. Here we found that hMSCs with a scaffold did not affect cell viability and osteogenic differentiation. We also investigated regenerative effect of hMSCs with the scaffold in a calvarial bone defect model. Formation of new bone was evaluated by micro-CT, histology and expression of osteogenic markers. The results clearly showed interesting evidence indicating that hMSCs with scaffold increased the formation of new bone and expression of osteogenic markers, compared to the empty and scaffold only groups. Overall, our results suggest that hMSCs with scaffold are suitable for stimulation of intense bone regeneration in critical-sized bone defects.
MeSH Terms
Figure
Reference
-
1. Koob S, Torio-Padron N, Stark GB, Hannig C, Stankovic Z, Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2011; 17(3-4):311–321. PMID: 20799886.
Article2. Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010; 5(6):e11177. PMID: 20567510.
Article3. Zong C, Xue D, Yuan W, Wang W, Shen D, Tong X, Shi D, Liu L, Zheng Q, Gao C, Wang J. Reconstruction of rat calvarial defects with human mesenchymal stem cells and osteoblast-like cells in poly-lactic-co-glycolic acid scaffolds. Eur Cell Mater. 2010; 20:109–120. PMID: 21249628.
Article4. Amorosa LF, Lee CH, Aydemir AB, Nizami S, Hsu A, Patel NR, Gardner TR, Navalgund A, Kim DG, Park SH, Mao JJ, Lee FY. Physiologic load-bearing characteristics of autografts, allografts, and polymer-based scaffolds in a critical sized segmental defect of long bone: an experimental study. Int J Nanomedicine. 2013; 8:1637–1643. PMID: 23637532.
Article5. Xing Z, Xue Y, Dånmark S, Schander K, Ostvold S, Arvidson K, Hellem S, Finne-Wistrand A, Albertsson AC, Mustafa K. Effect of endothelial cells on bone regeneration using poly(L-lactide-co-1,5-dioxepan-2-one) scaffolds. J Biomed Mater Res A. 2011; 96(2):349–357. PMID: 21171154.6. Zou D, Zhang Z, He J, Zhu S, Wang S, Zhang W, Zhou J, Xu Y, Huang Y, Wang Y, Han W, Zhou Y, Wang S, You S, Jiang X, Huang Y. Repairing critical-sized calvarial defects with BMSCs modified by a constitutively active form of hypoxia-inducible factor-1 and a phosphate cement scaffold. Biomaterials. 2011; 32(36):9707–9718. PMID: 21975460.
Article7. He X, Dziak R, Yuan X, Mao K, Genco R, Swihart M, Sarkar D, Li C, Wang C, Lu L, Andreadis S, Yang S. BMP2 genetically engineered MSCs and EPCs promote vascularized bone regeneration in rat critical-sized calvarial bone defects. PLoS One. 2013; 8(4):e60473. PMID: 23565253.
Article8. Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008; 83(5):723–730. PMID: 17898702.
Article9. Qi Y, Du Y, Li W, Dai X, Zhao T, Yan W. Cartilage repair using mesenchymal stem cell (MSC) sheet and MSCs-loaded bilayer PLGA scaffold in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2012.
Article10. Levi B, James AW, Nelson ER, Peng M, Wan DC, Commons GW, Lee M, Wu B, Longaker MT. Acute skeletal injury is necessary for human adipose-derived stromal cell-mediated calvarial regeneration. Plast Reconstr Surg. 2011; 127(3):1118–1129. PMID: 21364415.
Article11. Huang S, Wang Z. Platelet-rich plasma-derived growth factors promote osteogenic differentiation of rat muscle satellite cells: in vitro and in vivo studies. Cell Biol Int. 2012; 36(12):1195–1205. PMID: 22988823.12. Faghihi F, Baghaban Eslaminejad M. The effect of nano-scale topography on osteogenic differentiation of mesenchymal stem cells. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013.
Article13. Wang L, Rao RR, Stegemann JP. Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs. 2013; 197(5):333–343. PMID: 23571151.
Article14. Rampichová M, Chvojka J, Buzgo M, Prosecká E, Mikeš P, Vysloužilová L, Tvrdík D, Kochová P, Gregor T, Lukáš D, Amler E. Elastic three-dimensional poly (-caprolactone) nanofibre scaffold enhances migration, proliferation and osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2013; 46(1):23–37. PMID: 23216517.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cells in scaffold
- BONE REGENERATION WITH INJECTABLE MPEG-PCL DIBLOCK COPOLYMER AND BONE MARROW MESENCHYMAL STEM CELL
- Periosteum-Derived Mesenchymal Stem Cells and Scaffolds Transplanted into Long-Bone Defects of Rabbit
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Bone tissue engineering using PLLA/HA composite scaffold and bone marrow mesenchymal stem cell