Korean J Physiol Pharmacol.
2013 Oct;17(5):435-440. 10.4196/kjpp.2013.17.5.435.
Antiapoptotic Effect of Paricalcitol in Gentamicin-induced Kidney Injury
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju 501-757, Korea. skimw@chonnam.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwangju 501-757, Korea.
- KMID: 1500239
- DOI: http://doi.org/10.4196/kjpp.2013.17.5.435
Abstract
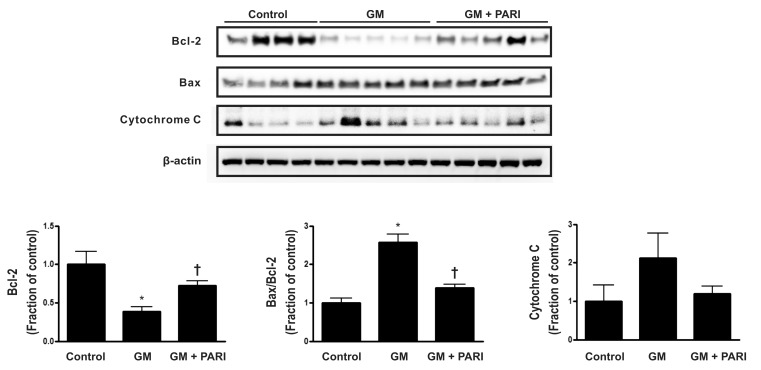
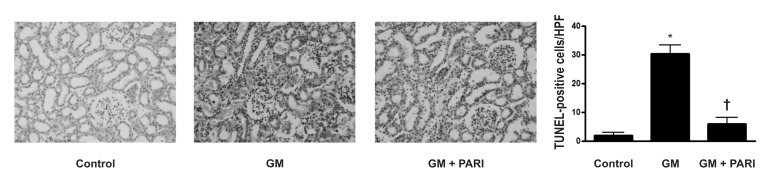
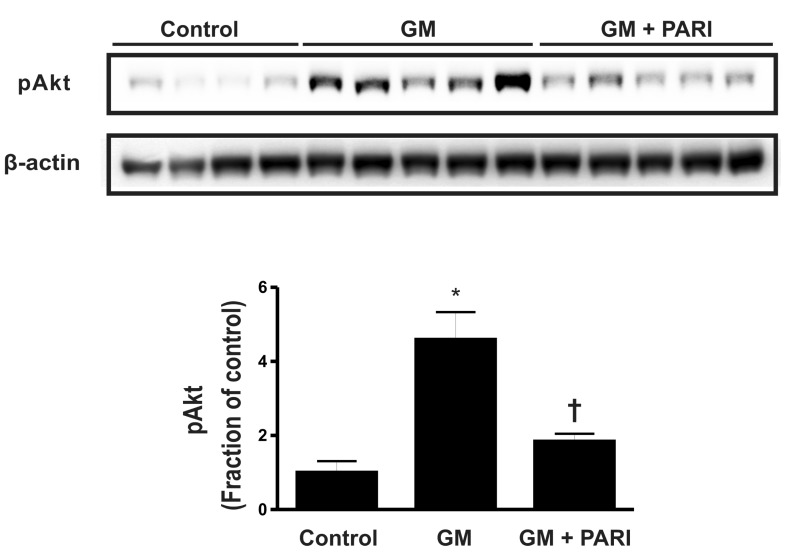
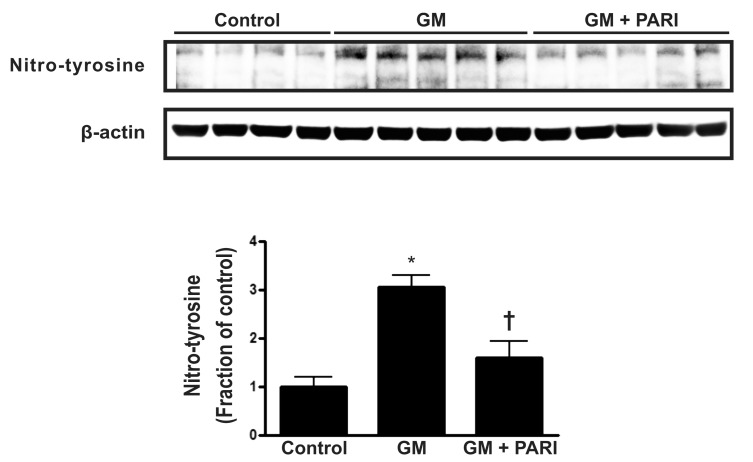
- While the anti-apoptotic effect of paricalcitol has been demonstrated in various animal models, it is not yet clear whether paricalcitol attenuates the apoptosis in gentamicin (GM)-induced kidney injury. We investigated the effect of paricalcitol on apoptotic pathways in rat kidneys damaged by GM. Rats were randomly divided into three groups: 1) Control group (n=8), where only vehicle was delivered, 2) GM group (n=10), where rats were treated with GM (150 mg/kg/day) for 7 days, 3) PARI group (n=10), where rats were co-treated with paricalcitol (0.2 microg/kg/day) and GM for 7 days. Paricalcitol attenuated renal dysfunction by GM administration in biochemical profiles. In terminal deoxynucleotidyl transferase dUTP nick end labeling staining, increased apoptosis was observed in GM group, which was reversed by paricalcitol co-treatment. Immunoblotting using protein samples from rat cortex/outer stripe of outer medulla showed increased Bax/Bcl-2 ratio and cleaved form of caspase-3 in GM group, both of which were reversed by paricalcitol. The phosphorylated Jun-N-terminal kinase (JNK) expression was increase in GM, which was counteracted by paricalcitol. The protein expression of p-Akt and nitro-tyrosine was also enhanced in GM-treated rats compared with control rats, which was reversed by paricalcitol co-treatment. Paricalcitol protects GM-induced renal injury by antiapoptotic mechanisms, including inhibition of intrinsic apoptosis pathway and JNK.
Keyword
MeSH Terms
Figure
Reference
-
1. Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011; 26:144–150. PMID: 20591815.
Article2. Martínez-Salgado C, Eleno N, Tavares P, Rodríguez-Barbero A, García-Criado J, Bolaños JP, López-Novoa JM. Involvement of reactive oxygen species on gentamicin-induced mesangial cell activation. Kidney Int. 2002; 62:1682–1692. PMID: 12371968.
Article3. Peyrou M, Hanna PE, Cribb AE. Cisplatin, gentamicin, and p-aminophenol induce markers of endoplasmic reticulum stress in the rat kidneys. Toxicol Sci. 2007; 99:346–353. PMID: 17567590.
Article4. Bae EH, Lee J, Ma SK, Kim IJ, Frøkiaer J, Nielsen S, Kim SY, Kim SW. Alpha-Lipoic acid prevents cisplatin-induced acute kidney injury in rats. Nephrol Dial Transplant. 2009; 24:2692–2700. PMID: 19376830.5. Park JW, Park CH, Kim IJ, Bae EH, Ma SK, Lee JU, Kim SW. Rho kinase inhibition by fasudil attenuates cyclosporine-induced kidney injury. J Pharmacol Exp Ther. 2011; 338:271–279. PMID: 21474569.
Article6. Wu X, Guo R, Chen P, Wang Q, Cunningham PN. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am J Physiol Renal Physiol. 2009; 297:F316–F326. PMID: 19420112.
Article7. Kelly KJ, Plotkin Z, Dagher PC. Guanosine supplementation reduces apoptosis and protects renal function in the setting of ischemic injury. J Clin Invest. 2001; 108:1291–1298. PMID: 11696573.
Article8. Bae EH, Cho S, Joo SY, Ma SK, Kim SH, Lee J, Kim SW. 4-Hydroxy-2-hexenal-induced apoptosis in human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2011; 26:3866–3873. PMID: 21771752.
Article9. Lee KE, Kim EY, Kim CS, Choi JS, Bae EH, Ma SK, Kim KK, Lee JU, Kim SW, Lee KE, Kim EY, Kim CS, Choi JS, Bae EH, Ma SK, Kim KK, Lee JU, Kim SW. Macrophage-stimulating protein attenuates gentamicin-induced inflammation and apoptosis in human renal proximal tubular epithelial cells. Biochem Biophys Res Commun. 2013; 434:527–533. PMID: 23583394.
Article10. Homsi E, Janino P, de Faria JB. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006; 69:1385–1392. PMID: 16557226.
Article11. Daemen MA, van 't Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999; 104:541–549. PMID: 10487768.
Article12. Guo R, Wang Y, Minto AW, Quigg RJ, Cunningham PN. Acute renal failure in endotoxemia is dependent on caspase activation. J Am Soc Nephrol. 2004; 15:3093–3102. PMID: 15579512.
Article13. García IM, Altamirano L, Mazzei L, Fornés M, Molina MN, Ferder L, Manucha W. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Renal Physiol. 2012; 302:F1595–F1605. PMID: 22492946.
Article14. Park JW, Cho JW, Joo SY, Kim CS, Choi JS, Bae EH, Ma SK, Kim SH, Lee J, Kim SW. Paricalcitol prevents cisplatin-induced renal injury by suppressing apoptosis and proliferation. Eur J Pharmacol. 2012; 683:301–309. PMID: 22449373.
Article15. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int. 2010; 77:1076–1085. PMID: 20237458.
Article16. Park JW, Bae EH, Kim IJ, Ma SK, Choi C, Lee J, Kim SW. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Renal Physiol. 2010; 298:F301–F313. PMID: 19940033.
Article17. Bae WK, Lee JU, Park JW, Bae EH, Ma SK, Kim SH, Kim SW, Bae WK, Lee JU, Park JW, Bae EH, Ma SK, Kim SH, Kim SW. Decreased expression of Na+/KX+-ATPase, NHE3, NBC1, AQP1 and OAT in gentamicin-induced nephropathy. Korean J Physiol Pharmacol. 2008; 12:331–336. PMID: 19967075.18. Lee J, Yoo KS, Kang DG, Kim SW, Choi KC. Gentamicin decreases the abundance of aquaporin water channels in rat kidney. Jpn J Pharmacol. 2001; 85:391–398. PMID: 11388643.
Article20. Hung RW, Chow AW. Dissecting the "end game": clinical relevance, molecular mechanisms and laboratory assessment of apoptosis. Clin Invest Med. 2004; 27:324–344. PMID: 15675113.21. Clemens MJ, van Venrooij WJ, van de Putte LB. Apoptosis and autoimmunity. Cell Death Differ. 2000; 7:131–133. PMID: 10858073.
Article22. Mao H, Li Z, Zhou Y, Li Z, Zhuang S, An X, Zhang B, Chen W, Nie J, Wang Z, Borkan SC, Wang Y, Yu X. HSP72 attenuates renal tubular cell apoptosis and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol. 2008; 295:F202–F214. PMID: 18417540.
Article23. Servais H, Ortiz A, Devuyst O, Denamur S, Tulkens PM, Mingeot-Leclercq MP. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis. 2008; 13:11–32. PMID: 17968659.
Article24. Oliver L, Vallette FM. The role of caspases in cell death and differentiation. Drug Resist Updat. 2005; 8:163–170. PMID: 15946892.
Article25. Lorz C, Justo P, Sanz AB, Egido J, Ortíz A. Role of Bcl-xL in paracetamol-induced tubular epithelial cell death. Kidney Int. 2005; 67:592–601. PMID: 15673306.
Article26. Hughes J, Gobe G. Identification and quantification of apoptosis in the kidney using morphology, biochemical and molecular markers. Nephrology (Carlton). 2007; 12:452–458. PMID: 17803468.
Article27. Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003; 304:437–444. PMID: 12729577.
Article28. Iverson SL, Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys. 2004; 423:37–46. PMID: 14989263.
Article29. Zanke BW, Boudreau K, Rubie E, Winnett E, Tibbles LA, Zon L, Kyriakis J, Liu FF, Woodgett JR. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996; 6:606–613. PMID: 8805279.
Article30. Park SJ, Jeong KS. Cell-type-specific activation of mitogen-activated protein kinases in PAN-induced progressive renal disease in rats. Biochem Biophys Res Commun. 2004; 323:1–8. PMID: 15351692.
Article31. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997; 91:231–241. PMID: 9346240.
Article32. Chen YC, Chen CH, Hsu YH, Chen TH, Sue YM, Cheng CY, Chen TW. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur J Pharmacol. 2011; 658:213–218. PMID: 21371463.
Article33. Lee KE, Kim EY, Kim CS, Choi JS, Bae EH, Ma SK, Park JS, Jung YD, Kim SH, Lee JU, Kim SW. Macrophage-stimulating protein attenuates hydrogen peroxide-induced apoptosis in human renal HK-2 cells. Eur J Pharmacol. 2013; 715:304–311. PMID: 23726950.
Article34. Runyan CE, Schnaper HW, Poncelet AC. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J Biol Chem. 2004; 279:2632–2639. PMID: 14610066.35. Hubchak SC, Sparks EE, Hayashida T, Schnaper HW. Rac1 promotes TGF-beta-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am J Physiol Renal Physiol. 2009; 297:F1316–F1323. PMID: 19726546.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Paricalcitol attenuates lipopolysaccharide-induced inflammation and apoptosis in proximal tubular cells through the prostaglandin Eâ‚‚ receptor EP4
- Anti-inflammatory and anti-apoptotic effects of paricalcitol in lipopolysaccharide-induced renal proximal tubular cell injury
- Paricalcitol attenuates indoxyl sulfate-induced apoptosis through the inhibition of MAPK, Akt, and NF-κB activation in HK-2 cells
- Intraperitoneal Paricalcitol Treatment for Secondary Hyperparathyroidism in CAPD patient: A Case Report
- Paricalcitol prevents MAPK pathway activation and inflammation in adriamycin-induced kidney injury in rats