J Pathol Transl Med.
2024 Sep;58(5):219-228. 10.4132/jptm.2024.07.12.
Paricalcitol prevents MAPK pathway activation and inflammation in adriamycin-induced kidney injury in rats
- Affiliations
-
- 1Laboratory of Renal Physiology, Department of Physiology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, Sao Paulo, Brazil
- 2Department of Pediatrics, Child Health Research Center, University of Virginia School of Medicine, Charlottesville, Virginia, United States of America
- 3Transplantation Immunobiology Laboratory, Department of Immunology, Institute of Biomedical Sciences, University of Sao Paulo, Sao Paulo, Brazil
- 4Laboratory of Renal Pathology, Division of Nephrology, Department of Internal Medicine, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, Sao Paulo, Brazil
- KMID: 2559067
- DOI: http://doi.org/10.4132/jptm.2024.07.12
Abstract
- Background
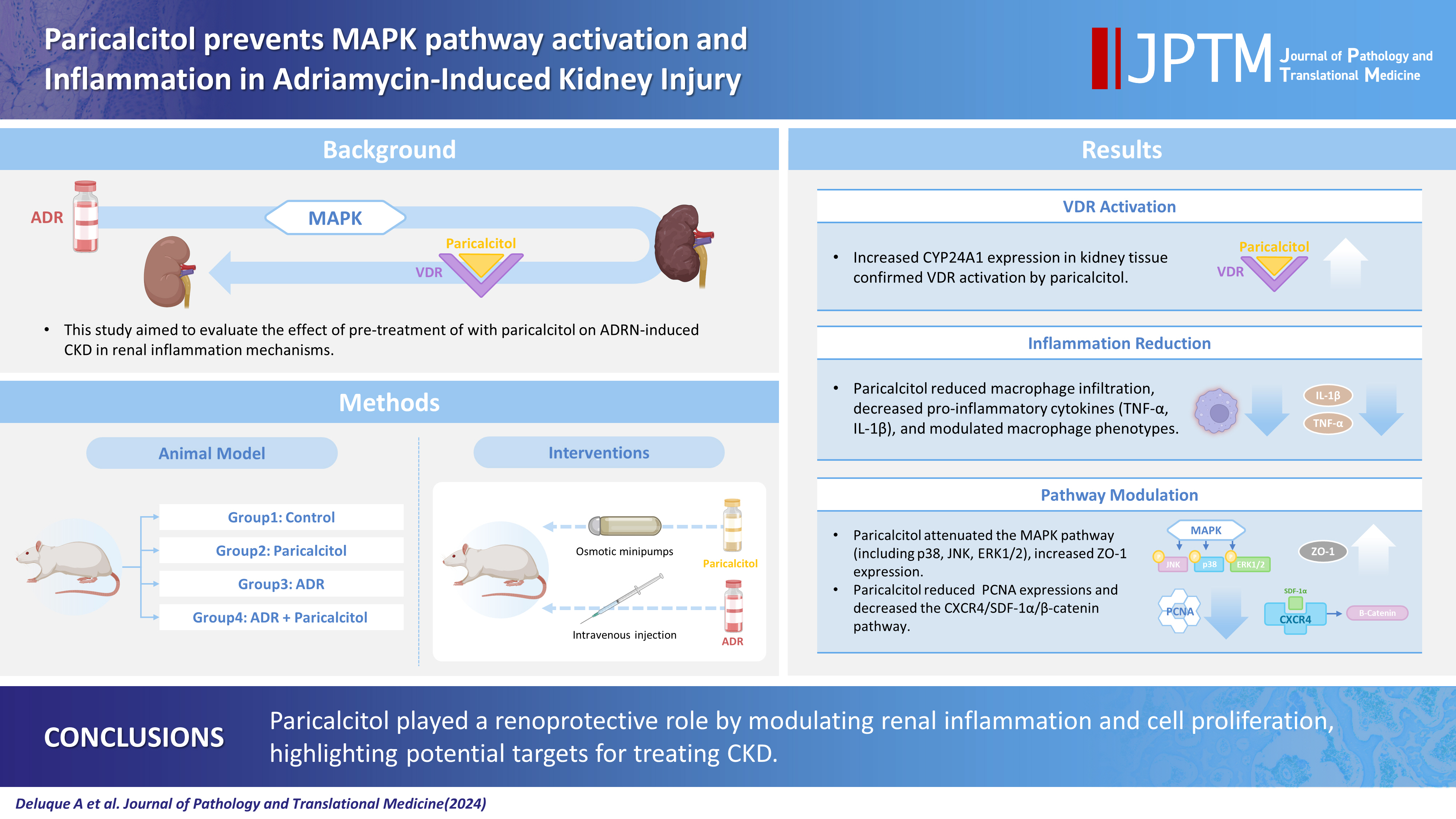
Activation of the mitogen-activated protein kinase (MAPK) pathway induces uncontrolled cell proliferation in response to inflammatory stimuli. Adriamycin (ADR)-induced nephropathy (ADRN) in rats triggers MAPK activation and pro-inflammatory mechanisms by increasing cytokine secretion, similar to chronic kidney disease (CKD). Activation of the vitamin D receptor (VDR) plays a crucial role in suppressing the expression of inflammatory markers in the kidney and may contribute to reducing cellular proliferation. This study evaluated the effect of pre-treatment with paricalcitol on ADRN in renal inflammation mechanisms.
Methods
Male Sprague-Dawley rats were implanted with an osmotic minipump containing activated vitamin D (paricalcitol, Zemplar, 6 ng/day) or vehicle (NaCl 0.9%). Two days after implantation, ADR (Fauldoxo, 3.5 mg/kg) or vehicle (NaCl 0.9%) was injected. The rats were divided into four experimental groups: control, n = 6; paricalcitol, n = 6; ADR, n = 7 and, ADR + paricalcitol, n = 7.
Results
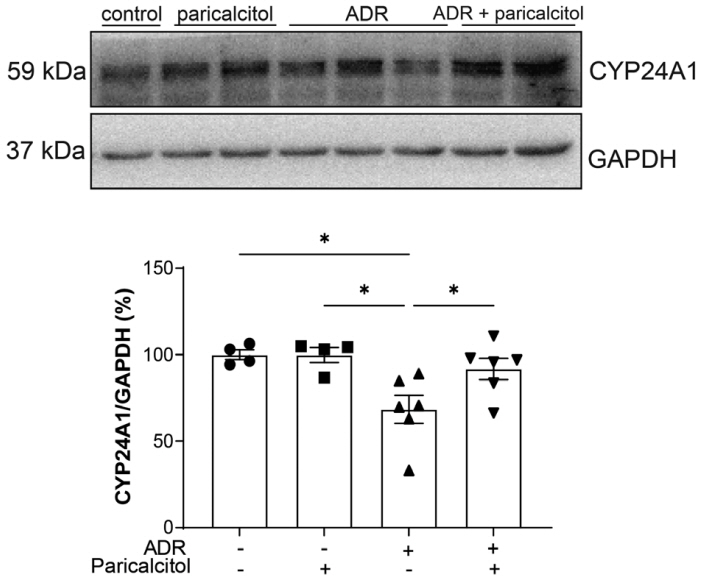
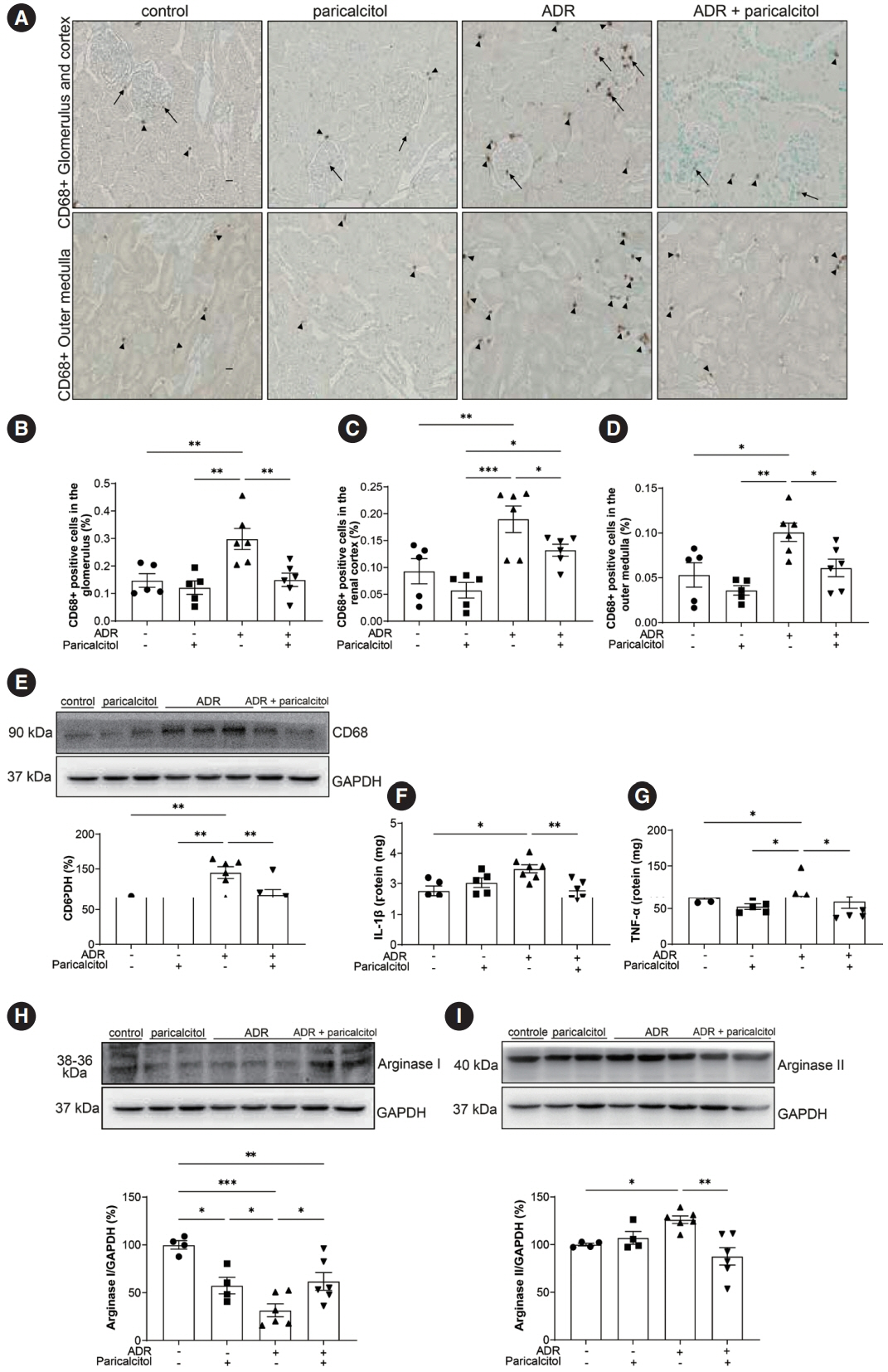
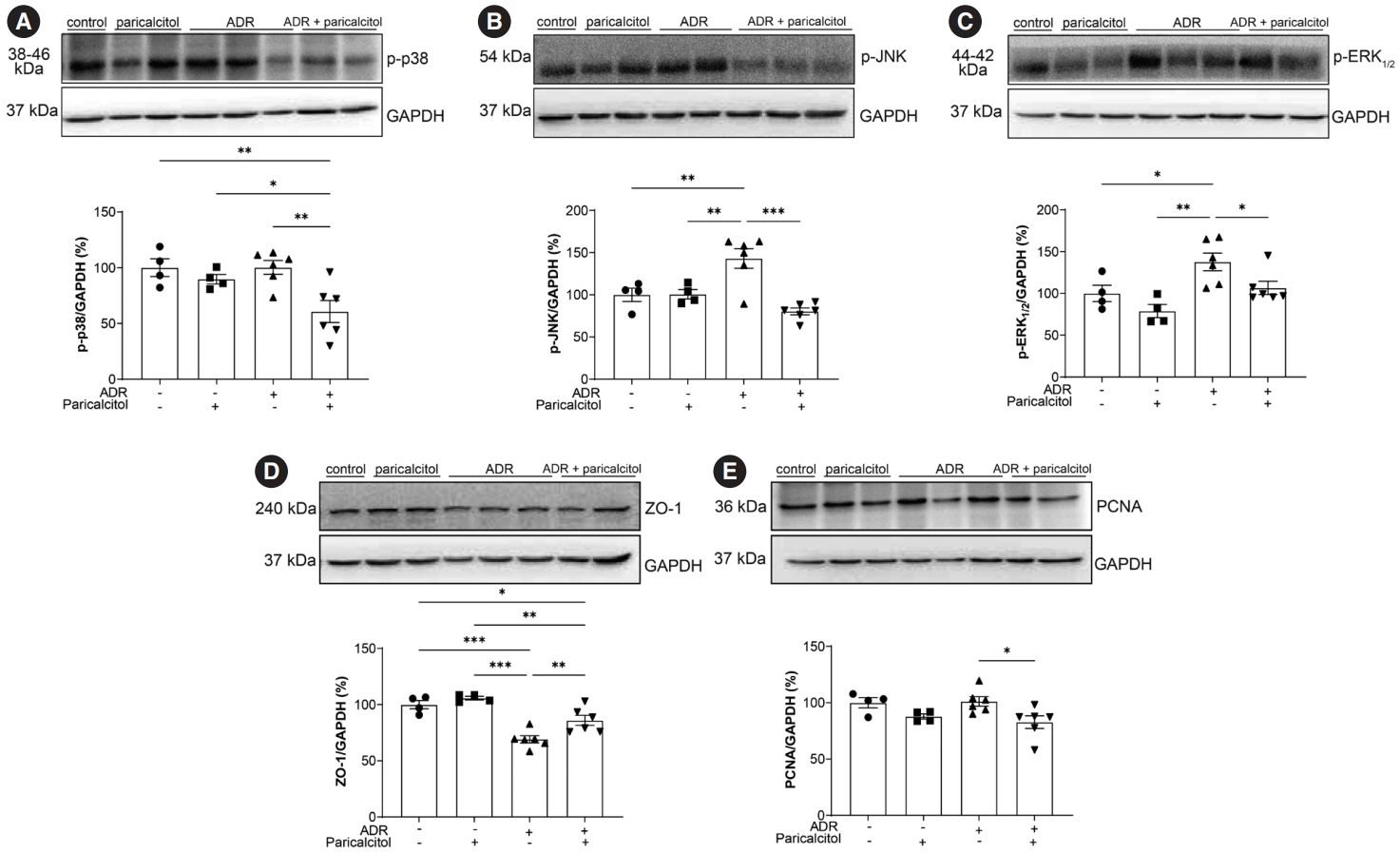
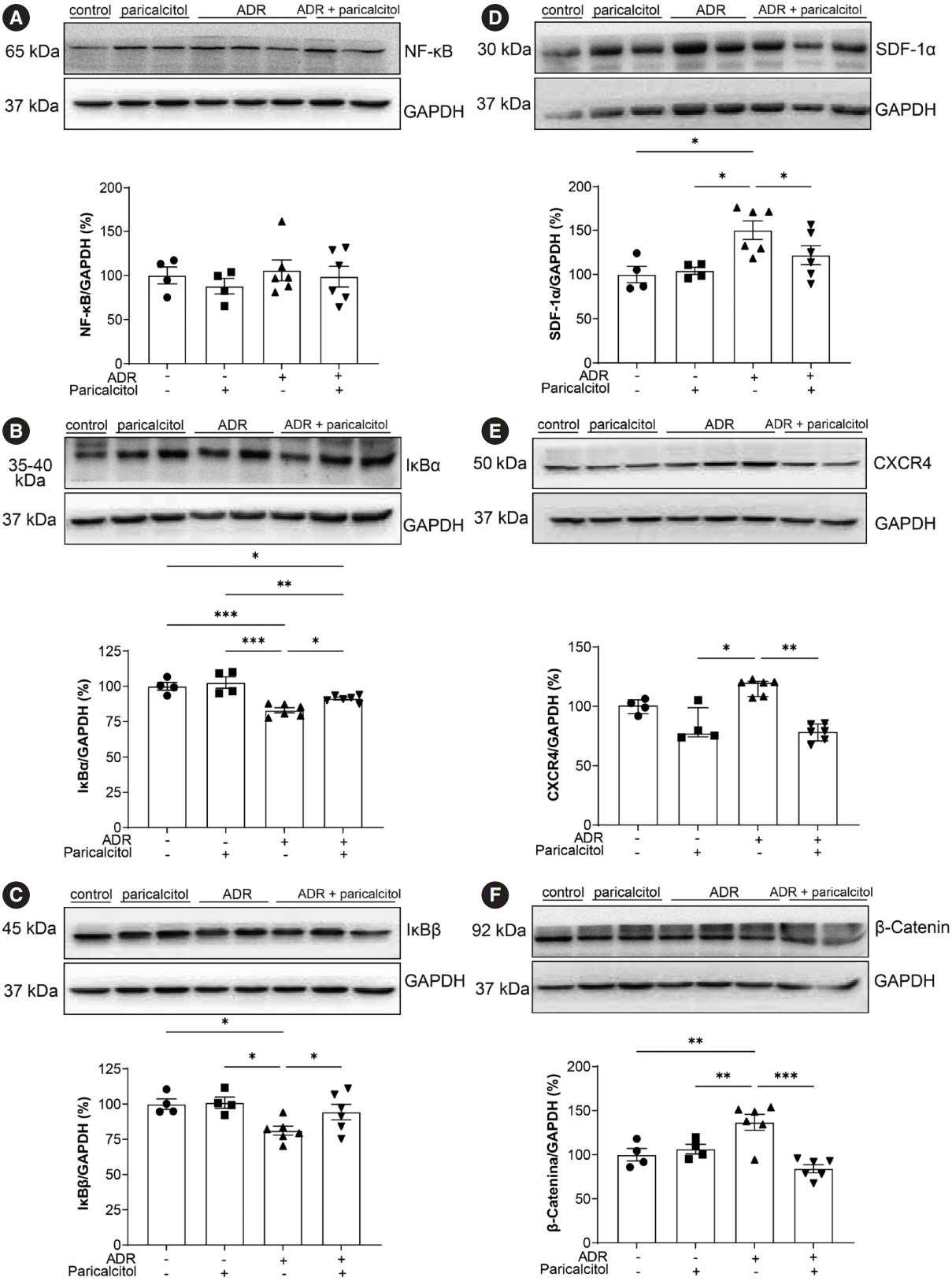
VDR activation was demonstrated by increased CYP24A1 in renal tissue. Paricalcitol prevented macrophage infiltration in the glomeruli, cortex, and outer medulla, prevented secretion of tumor necrosis factor-α, and interleukin-1β, increased arginase I and decreased arginase II tissue expressions, effects associated with attenuation of MAPK pathways, increased zonula occludens-1, and reduced cell proliferation associated with proliferating cell nuclear antigen expression. Paricalcitol treatment decreased the stromal cell-derived factor 1α/chemokine C-X-C receptor type 4/β-catenin pathway.
Conclusions
Paricalcitol plays a renoprotective role by modulating renal inflammation and cell proliferation. These results highlight potential targets for treating CKD.
Figure
Reference
-
References
1. Deluque AL, Oliveira BM, Souza CS, et al. Paricalcitol improves the angiopoietin/Tie-2 and VEGF/VEGFR2 signaling pathways in adriamycin-induced nephropathy. Nutrients. 2022; 14:5316.2. Hua W, Ten Dijke P, Kostidis S, Giera M, Hornsveld M. TGFbeta-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer. Cell Mol Life Sci. 2020; 77:2103–23.3. Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014; 10:493–503.4. Jourde-Chiche N, Fakhouri F, Dou L, et al. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019; 15:87–108.5. Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019; 15:144–58.6. Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant. 2015; 30:706–12.7. Wu Q, Li W, Zhao J, et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed Pharmacother. 2021; 137:111308.8. Li W, He W, Xia P, et al. Total extracts of Abelmoschus manihot L. attenuates adriamycin-induced renal tubule injury via suppression of ROS-ERK1/2-mediated NLRP3 inflammasome activation. Front Pharmacol. 2019; 10:567.9. Sureshbabu A, Muhsin SA, Choi ME. TGF-beta signaling in the kidney: profibrotic and protective effects. Am J Physiol Renal Physiol. 2016; 310:F596–F606.10. Martinez-Arias L, Panizo S, Alonso-Montes C, et al. Effects of calcitriol and paricalcitol on renal fibrosis in CKD. Nephrol Dial Transplant. 2021; 36:793–803.11. Egido J, Martinez-Castelao A, Bover J, et al. The pleiotropic effects of paricalcitol: beyond bone-mineral metabolism. Nefrologia. 2016; 36:10–8.12. Salanova Villanueva L, Gil Giraldo Y, Santos Sanchez-Rey B, Aguilera Peralta A. Paricalcitol regulatory effect on inflammatory, fibrotic and anticalcificating parameters in renal patiente: far beyond mineral bone disease regulation. Nefrologia (Engl Ed). 2020; 40:171–9.13. Oliveira BM, de Almeida LF, Deluque AL, et al. Calcitriol reduces the inflammation, endothelial damage and oxidative stress in AKI caused by cisplatin. Int J Mol Sci. 2022; 23:15877.14. Deluque AL, de Almeida LF, Francescato HD, et al. Effect of calcitriol on the renal microvasculature differentiation disturbances induced by AT(1) blockade during nephrogenesis in rats. Front Med (Lausanne). 2020; 7:23.15. Souza CS, Deluque AL, Oliveira BM, et al. Vitamin D deficiency contributes to the diabetic kidney disease progression via increase ZEB1/ZEB2 expressions. Nutr Diabetes. 2023; 13:9.16. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014; 21:319–29.17. Reis NG, Francescato HD, de Almeida LF, Silva C, Costa RS, Coimbra TM. Protective effect of calcitriol on rhabdomyolysis-induced acute kidney injury in rats. Sci Rep. 2019; 9:7090.18. Agarwal R, Acharya M, Tian J, et al. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005; 68:2823–8.19. Huen SC, Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol. 2017; 79:449–69.20. Li Z, Wang L, Ren Y, et al. Arginase: shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022; 8:413.21. Sui X, Kong N, Ye L, et al. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014; 344:174–9.22. Zhou Y, Wu Q, Du Z, et al. Verbena attenuates adriamycin-induced renal tubular injury via inhibition of ROS-ERK1/2-NLRP3 signal pathway. Evid Based Complement Alternat Med. 2022; 2022:7760945.23. Chung S, Kim S, Kim M, et al. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin. PLoS One. 2017; 12:e0181757.24. de Almeida LF, Francescato HD, da Silva CG, Costa RS, Coimbra TM. Calcitriol reduces kidney development disorders in rats provoked by losartan administration during lactation. Sci Rep. 2017; 7:11472.25. Diaz-Coranguez M, Liu X, Antonetti DA. Tight junctions in cell proliferation. Int J Mol Sci. 2019; 20:5972.26. He L, Zhou Z, Shao Y, et al. Bradykinin potentially stimulates cell proliferation in rabbit corneal endothelial cells through the ZO-1/ZONAB pathway. Int J Mol Med. 2018; 42:71–80.27. Raggi C, Luciani A, Nevo N, Antignac C, Terryn S, Devuyst O. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum Mol Genet. 2014; 23:2266–78.28. Li X, Liu J, Zhao Y, et al. 1,25-dihydroxyvitamin D3 ameliorates lupus nephritis through inhibiting the NF-kappaB and MAPK signalling pathways in MRL/lpr mice. BMC Nephrol. 2022; 23:243.29. Wang Q, He Y, Shen Y, et al. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J Biol Chem. 2014; 289:11681–94.30. Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016; 12:325–38.31. Mo H, Ren Q, Song D, et al. CXCR4 induces podocyte injury and proteinuria by activating beta-catenin signaling. Theranostics. 2022; 12:767–81.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiapoptotic Effect of Paricalcitol in Gentamicin-induced Kidney Injury
- Paricalcitol attenuates indoxyl sulfate-induced apoptosis through the inhibition of MAPK, Akt, and NF-κB activation in HK-2 cells
- Paricalcitol attenuates lipopolysaccharide-induced inflammation and apoptosis in proximal tubular cells through the prostaglandin Eâ‚‚ receptor EP4
- D-Limonene mitigate myocardial injury in rats through MAPK/ ERK/NF-κB pathway inhibition
- The p38 mitogen-activated protein kinase is involved in stress-induced phospholipase D activation in vascular smooth muscle cells