Immune Netw.
2010 Oct;10(5):173-180. 10.4110/in.2010.10.5.173.
Cell to Cell Interaction Can Activate Membrane-bound APRIL Which Are Expressed on Inflammatory Macrophages
- Affiliations
-
- 1From School of Life Sciences and Biotechnology, Kyungpook National University, Daegu 702-701, Korea. whl@knu.ac.kr
- 2Department of Pharmacology, Brain Science and Engineering Institute, College of Medicine, Kyungpook National University, Daegu 702-701, Korea.
- KMID: 1456221
- DOI: http://doi.org/10.4110/in.2010.10.5.173
Abstract
- BACKGROUND
APRIL, originally known as a cytokine involved in B cell survival, is now known to regulate the inflammatory activation of macrophages. Although the signal initiated from APRIL has been demonstrated, its role in cellular activation is still not clear due to the presence of BAFF, a closely related member of TNF superfamily, which share same receptors (TACI and BCMA) with APRIL.
METHODS
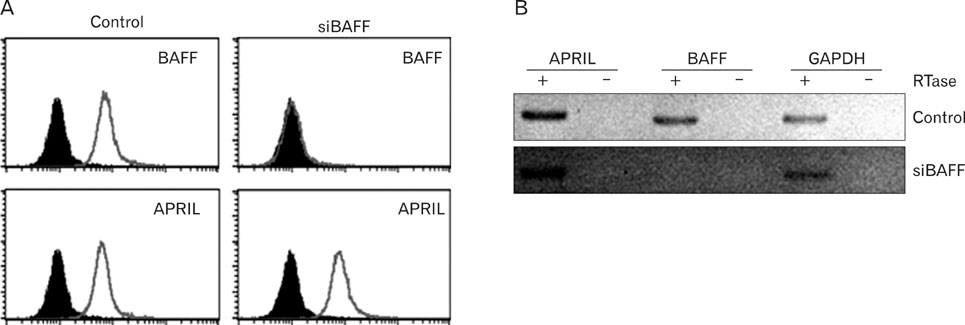
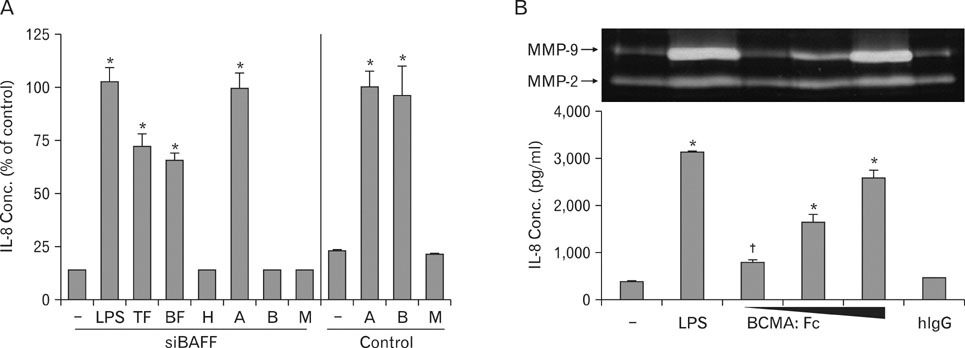
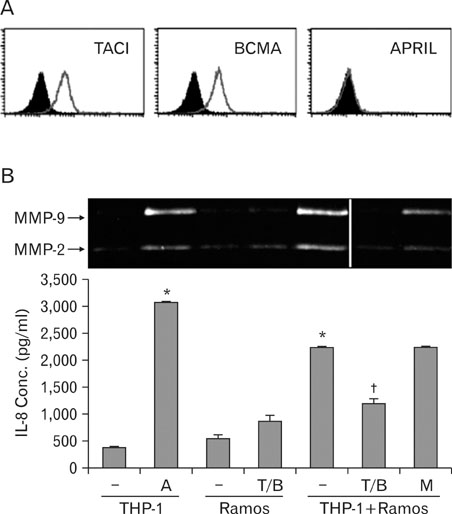
Through transfection of siRNA, BAFF-deficient THP-1 cells (human macrophage-like cells) were generated and APRIL-mediated inflammatory activities were tested. The expression patterns of APRIL were also tested in vivo.
RESULTS
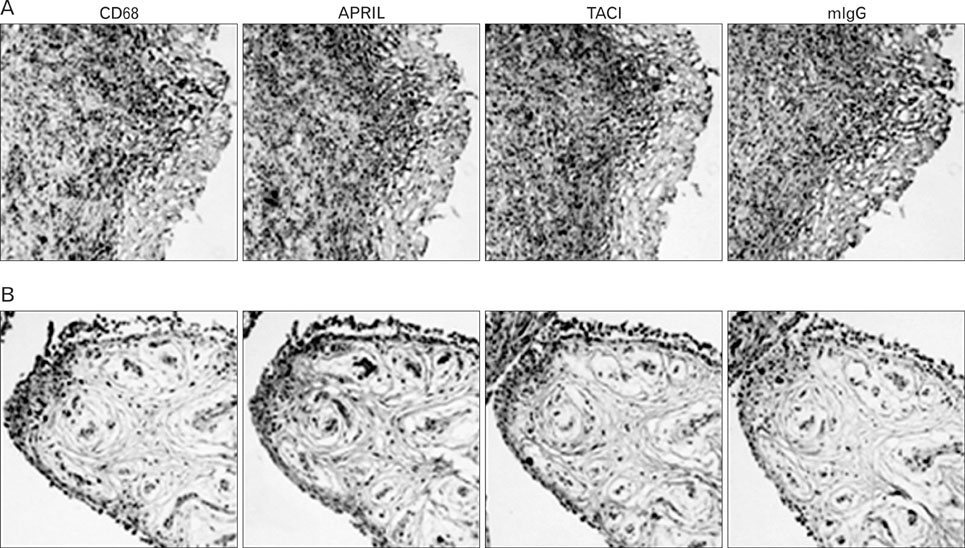
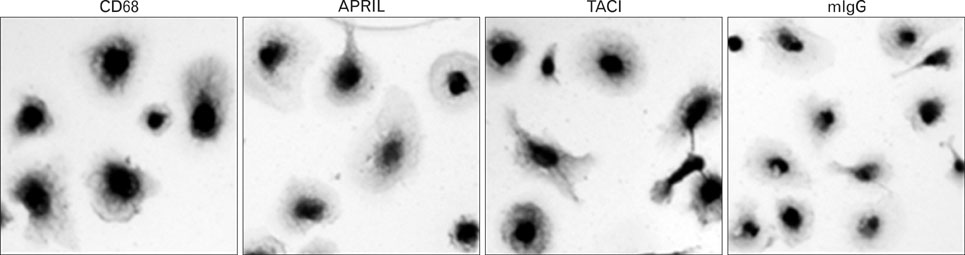
BAFF-deficient THP-1 cells responded to APRIL-stimulating agents such as monoclonal antibody against APRIL and soluble form of TACI or BCMA. Furthermore, co-incubation of the siBAFF-deficient THP-1 cells with a human B cell line (Ramos) resulted in an activation of THP-1 cells which was dependent on interactions between APRIL and TACI/BCMA. Immunohistochemical analysis of human pathologic samples detected the expression of both APRIL and TACI in macrophage-rich areas. Additionally, human macrophage primary culture expressed APRIL on the cell surface.
CONCLUSION
These observations indicate that APRIL, which is expressed on macrophages in pathologic tissues with chronic inflammation, may mediate activation signals through its interaction with its counterparts via cell-to-cell interaction.
Keyword
MeSH Terms
Figure
Reference
-
1. Hahne M, Kataoka T, Schröter M, Hofmann K, Irmler M, Bodmer JL, Schneider P, Bornand T, Holler N, French LE, Sordat B, Rimoldi D, Tschopp J. APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. 1998. 188:1185–1190.
Article2. Kelly K, Manos E, Jensen G, Nadauld L, Jones DA. APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res. 2000. 60:1021–1027.3. Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999. 65:680–683.
Article4. Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol. 2003. 21:231–264.
Article5. Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003. 198:937–945.
Article6. Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol Immunol. 2005. 42:763–772.
Article7. Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006. 5:235–246.
Article8. Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005. 17:282–289.
Article9. Jeon ST, Kim WJ, Lee SM, Lee MY, Park SB, Lee SH, Kim IS, Suk K, Choi BK, Choi EM, Kwon BS, Lee WH. Reverse signaling through BAFF differentially regulates the expression of inflammatory mediators and cytoskeletal movements in THP-1 cells. Immunol Cell Biol. 2010. 88:148–156.
Article10. Lee SM, Jeon ST, Kim WJ, Suk KH, Lee WH. Macrophages express membrane bound form of APRIL that can generate immunomodulatory signals. Immunology. 2010. 131:350–356.
Article11. Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H, Kwon BS, Park JE. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001. 21:2004–2010.
Article12. Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Netw. 2004. 4:116–122.
Article13. Colli S, Eligini S, Lalli M, Camera M, Paoletti R, Tremoli E. Vastatins inhibit tissue factor in cultured human macrophages. A novel mechanism of protection against atherothrombosis. Arterioscler Thromb Vasc Biol. 1997. 17:265–272.14. Lesnik P, Rouis M, Skarlatos S, Kruth HS, Chapman MJ. Uptake of exogenous free cholesterol induces upregulation of tissue factor expression in human monocyte-derived macrophages. Proc Natl Acad Sci U S A. 1992. 89:10370–10374.
Article15. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.
Article16. Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996. 7:330–335.
Article17. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.18. Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993. 81:1607–1613.
Article19. Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999. 26:717–719.20. Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996. 39:115–124.
Article21. Yanni G, Whelan A, Feighery C, Bresnihan B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann Rheum Dis. 1994. 53:39–44.
Article22. Tamada K, Shimozaki K, Chapoval AI, Zhai Y, Su J, Chen SF, Hsieh SL, Nagata S, Ni J, Chen L. LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol. 2000. 164:4105–4110.
Article23. van Lent PL, Holthuysen AE, van den Bersselaar LA, van Rooijen N, Joosten LA, van de Loo FA, van de Putte LB, van den Berg WB. Phagocytic lining cells determine local expression of inflammation in type II collagen-induced arthritis. Arthritis Rheum. 1996. 39:1545–1555.
Article24. Van Lent PL, Holthuysen AE, Van Rooijen N, Van De Putte LB, Van Den Berg WB. Local removal of phagocytic synovial lining cells by clodronate-liposomes decreases cartilage destruction during collagen type II arthritis. Ann Rheum Dis. 1998. 57:408–413.
Article25. Kolfschoten GM, Pradet-Balade B, Hahne M, Medema JP. TWE-PRIL; a fusion protein of TWEAK and APRIL. Biochem Pharmacol. 2003. 66:1427–1432.
Article26. Pradet-Balade B, Medema JP, López-Fraga M, Lozano JC, Kolfschoten GM, Picard A, Martínez-A C, Garcia-Sanz JA, Hahne M. An endogenous hybrid mRNA encodes TWE-PRIL, a functional cell surface TWEAK-APRIL fusion protein. EMBO J. 2002. 21:5711–5720.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Therapy Applying Membrane-bound Form of Cytokines
- Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
- Single Cell Transcriptomic Re-analysis of Immune Cells in Bronchoalveolar Lavage Fluids Reveals the Correlation of B Cell Characteristics and Disease Severity of Patients with SARS-CoV-2 Infection
- Murine pro-tumor necrosis factor expressed in Saccharomyces cerevisiae HF7c localizes to membrane/particulate
- Mechanisms of Glucose Uptake in Cancer Tissue