Immune Netw.
2009 Oct;9(5):158-168. 10.4110/in.2009.9.5.158.
Tumor Therapy Applying Membrane-bound Form of Cytokines
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon 305-764, Korea. young@cnu.ac.kr
- KMID: 1474577
- DOI: http://doi.org/10.4110/in.2009.9.5.158
Abstract
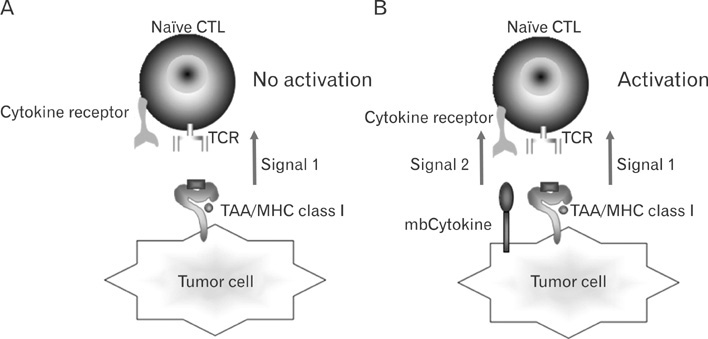
- Tumor therapy using cytokines has been developed for last two decades. Several recombinant cytokines and tumor cell vaccines produced by cytokine gene transfer have been in clinical trials, but several side effects hamper routine clinical applications. Many cytokines are originally expressed as membrane-bound form and then processed to secretory form exerting paracrine effects. Though functional differences of these two types of cytokines are elusive yet, the membrane-bound form of cytokine may exert its effects on restricted target cells as a juxtacrine, which are in physical contacts. With the efforts to improve antitumor activities of cytokines in cancer patients, developing new strategies to alleviate life-threatening side effects became an inevitable goal of tumor immunologists. Among these, tumor cell vaccines expressing cytokines as membrane-bound form on tumor cell surface have been developed by genetic engineering techniques with the hope of selective stimulation of the target cells that are in cell-to-cell contacts. In this review, recent progress of tumor cell vaccines expressing membrane-bound form of cytokines will be discussed.
Keyword
Figure
Cited by 3 articles
-
Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
Hoyong Lim, Seon Ah Do, Sang Min Park, Young Sang Kim
Immune Netw. 2013;13(2):63-69. doi: 10.4110/in.2013.13.2.63.Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
Van Anh Do Thi, Sang Min Park, Hayyoung Lee, Young Sang Kim
Immune Netw. 2018;18(1):e12. doi: 10.4110/in.2018.18.e12.Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
Van Anh Do Thi, Sang Min Park, Hayyoung Lee, Young Sang Kim
Immune Netw. 2018;18(1):. doi: 10.4110/in.2018.18.e12.
Reference
-
1. Tepper RI, Mule JJ. Experimental and clinical studies of cytokine gene-modified tumor cells. Hum Gene Ther. 1994. 5:153–164.
Article2. Blankenstein T, Rowley DA, Schreiber H. Cytokines and cancer: experimental systems. Curr Opin Immunol. 1991. 3:694–698.
Article3. Colombo MP, Forni G. Cytokine gene transfer in tumor inhibition and tumor therapy: where are we now? Immunol Today. 1994. 15:48–51.
Article4. Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993. 90:3539–3543.
Article5. Zier K, Gansbacher B, Salvadori S. Preventing abnormalities in signal transduction of T cells in cancer: the promise of cytokine gene therapy. Immunol Today. 1996. 17:39–45.
Article6. Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996. 170:101–110.7. Kurt-Jones EA, Fiers W, Pober JS. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987. 139:2317–2324.8. Musso T, Calosso L, Zucca M, Millesimo M, Ravarino D, Giovarelli M, Malavasi F, Ponzi AN, Paus R, Bulfone-Paus S. Human monocytes constitutively express membrane-bound, biologically active, and interferon-gamma-upregulated interleukin-15. Blood. 1999. 93:3531–3539.
Article9. Cerretti DP, Wignall J, Anderson D, Tushinski RJ, Gallis BM, Stya M, Gillis S, Urdal DL, Cosman D. Human macrophage-colony stimulating factor: alternative RNA and protein processing from a single gene. Mol Immunol. 1988. 25:761–770.
Article10. Lyman SD, James L, Escobar S, Downey H, de Vries P, Brasel K, Stocking K, Beckmann MP, Copeland NG, Cleveland LS, et al. Identification of soluble and membrane-bound isoforms of the murine flt3 ligand generated by alternative splicing of mRNAs. Oncogene. 1995. 10:149–157.11. Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988. 53:45–53.
Article12. Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin beta, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993. 72:847–856.
Article13. Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997. 385:640–644.
Article14. Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001. 194:629–644.
Article15. Assenmacher M, Scheffold A, Schmitz J, Segura Checa JA, Miltenyi S, Radbruch A. Specific expression of surface interferon-gamma on interferon-gamma producing T cells from mouse and man. Eur J Immunol. 1996. 26:263–267.
Article16. Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975. 72:3666–3670.
Article17. Li X, Fan H. Loss of ectodomain shedding due to mutations in the metalloprotease and cysteine-rich/disintegrin domains of the tumor necrosis factor-alpha converting enzyme (TACE). J Biol Chem. 2004. 279:27365–27375.
Article18. Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002. 34:1–5.19. Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989. 7:445–480.
Article20. Aversa G, Punnonen J, de Vries JE. The 26-kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J Exp Med. 1993. 177:1575–1585.
Article21. Birkland TP, Sypek JP, Wyler DJ. Soluble TNF and membrane TNF expressed on CD4+ T lymphocytes differ in their ability to activate macrophage antiLeishmanial defense. J Leukoc Biol. 1992. 51:296–299.
Article22. Mueller C, Corazza N, Trachsel-Løseth S, Eügster HP, Bühler-Jungo M, Brunner T, Imboden MA. Noncleavable transmembrane mouse tumor necrosis factor-alpha (TNFalpha) mediates effects distinct from those of wild-type TNFalpha in vitro and in vivo. J Biol Chem. 1999. 274:38112–38118.
Article23. Fichtner I, Lemm M, Becker M, Tanneberger S. Determination of antineoplastic activity and toxicity of tumor necrosis factor (TNF) in animal experiments. Correlation to clinical findings. Neoplasma. 1990. 37:301–315.24. Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994. 45:491–503.25. Marr RA, Addison CL, Snider D, Muller WJ, Gauldie J, Graham FL. Tumour immunotherapy using an adenoviral vector expressing a membrane-bound mutant of murine TNF alpha. Gene Ther. 1997. 4:1181–1188.
Article26. Li Q, Li L, Shi W, Jiang X, Xu Y, Gong F, Zhou M, Edwards CK 3rd, Li Z. Mechanism of action differences in the antitumor effects of transmembrane and secretory tumor necrosis factor-alpha in vitro and in vivo. Cancer Immunol Immunother. 2006. 55:1470–1479.
Article27. Rieger R, Whitacre D, Cantwell MJ, Prussak C, Kipps TJ. Chimeric form of tumor necrosis factor-alpha has enhanced surface expression and antitumor activity. Cancer Gene Ther. 2009. 16:53–64.
Article28. Burgess AW, Camakaris J, Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977. 252:1998–2003.
Article29. Pluznik DH, Sachs L. The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res. 1966. 43:553–563.
Article30. Witmer-Pack MD, Olivier W, Valinsky J, Schuler G, Steinman RM. Granulocyte/macrophage colony-stimulating factor is essential for the viability and function of cultured murine epidermal Langerhans cells. J Exp Med. 1987. 166:1484–1498.
Article31. Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000. 60:3239–3246.32. Soo Hoo W, Lundeen KA, Kohrumel JR, Pham NL, Brostoff SW, Bartholomew RM, Carlo DJ. Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J Immunol. 1999. 162:7343–7349.33. Yei S, Bartholomew RM, Pezzoli P, Gutierrez A, Gouveia E, Bassett D, Soo Hoo W, Carlo DJ. Novel membrane-bound GM-CSF vaccines for the treatment of cancer: generation and evaluation of mbGM-CSF mouse B16F10 melanoma cell vaccine. Gene Ther. 2002. 9:1302–1311.
Article34. el-Shami KM, Tzehoval E, Vadai E, Feldman M, Eisenbach L. Induction of antitumor immunity with modified autologous cells expressing membrane-bound murine cytokines. J Interferon Cytokine Res. 1999. 19:1391–1401.
Article35. Ling X, Wang Y, Dietrich MF, Andreeff M, Arlinghaus RB. Vaccination with leukemia cells expressing cell-surface-associated GM-CSF blocks leukemia induction in immunocompetent mice. Oncogene. 2006. 25:4483–4490.
Article36. Douglass TG, Driggers L, Zhang JG, Hoa N, Delgado C, Williams CC, Dan Q, Sanchez R, Jeffes EW, Wepsic HT, Myers MP, Koths K, Jadus MR. Macrophage colony stimulating factor: not just for macrophages anymore! A gateway into complex biologies. Int Immunopharmacol. 2008. 8:1354–1376.
Article37. Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ, Aebersold P, Leitman S, Linehan WM, Seipp CA, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993. 85:622–632.
Article38. Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000. 6:Suppl 1. 55–57.39. Assier E, Jullien V, Lefort J, Moreau JL, Di Santo JP, Vargaftig BB, Lapa e Silva JR, Theze J. NK cells and polymorphonuclear neutrophils are both critical for IL-2-induced pulmonary vascular leak syndrome. J Immunol. 2004. 172:7661–7668.
Article40. Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001. 24:287–293.
Article41. Chen B, Timiryasova TM, Gridley DS, Andres ML, Dutta-Roy R, Fodor I. Evaluation of cytokine toxicity induced by vaccinia virus-mediated IL-2 and IL-12 antitumour immunotherapy. Cytokine. 2001. 15:305–314.
Article42. Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998. 83:797–805.
Article43. Lotze MT, Matory YL, Rayner AA, Ettinghausen SE, Vetto JT, Seipp CA, Rosenberg SA. Clinical effects and toxicity of interleukin-2 in patients with cancer. Cancer. 1986. 58:2764–2772.
Article44. Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003. 349:427–434.
Article45. Mizoguchi H, O'Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992. 258:1795–1798.
Article46. Salvadori S, Gansbacher B, Pizzimenti AM, Zier KS. Abnormal signal transduction by T cells of mice with parental tumors is not seen in mice bearing IL-2-secreting tumors. J Immunol. 1994. 153:5176–5182.47. Fearon ER, Pardoll DM, Itaya T, Golumbek P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B, Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990. 60:397–403.
Article48. Gansbacher B, Zier K, Daniels B, Cronin K, Bannerji R, Gilboa E. Interleukin 2 gene transfer into tumor cells abrogates tumorigenicity and induces protective immunity. J Exp Med. 1990. 172:1217–1224.
Article49. Bannerji R, Arroyo CD, Cordon-Cardo C, Gilboa E. The role of IL-2 secreted from genetically modified tumor cells in the establishment of antitumor immunity. J Immunol. 1994. 152:2324–2332.50. Allione A, Consalvo M, Nanni P, Lollini PL, Cavallo F, Giovarelli M, Forni M, Gulino A, Colombo MP, Dellabona P, et al. Immunizing and curative potential of replicating and nonreplicating murine mammary adenocarcinoma cells engineered with interleukin (IL)-2, IL-4, IL-6, IL-7, IL-10, tumor necrosis factor alpha, granulocyte-macrophage colony-stimulating factor, and gamma-interferon gene or admixed with conventional adjuvants. Cancer Res. 1994. 54:6022–6026.51. Lollini PL, Forni G. Cancer immunoprevention: tracking down persistent tumor antigens. Trends Immunol. 2003. 24:62–66.
Article52. Tjuvajev J, Gansbacher B, Desai R, Beattie B, Kaplitt M, Matei C, Koutcher J, Gilboa E, Blasberg R. RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin-2 and interferon-gamma. Cancer Res. 1995. 55:1902–1910.53. Saparov A, Wagner FH, Zheng R, Oliver JR, Maeda H, Hockett RD, Weaver CT. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 1999. 11:271–280.
Article54. Nizard P, Gross DA, Babon A, Chenal A, Beaumelle B, Kosmatopoulos K, Gillet D. Anchoring cytokines to tumor cells for the preparation of anticancer vaccines without gene transfection in mice. J Immunother. 2003. 26:63–71.55. Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE, Wei Y. Synergistic anti-tumor effect of glycosylphosphatidylinositol-anchored IL-2 and IL-12. J Gene Med. 2004. 6:777–785.
Article56. Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE, Wei Y. Glycoinositol phospholipid-anchored interleukin 2 but not secreted interleukin 2 inhibits melanoma tumor growth in mice. Mol Cancer Ther. 2002. 1:1019–1024.57. Sonn CH, Yoon HR, Seong IO, Chang MR, Kim YC, Kang HC, Suh SC, Kim YS. MethA fibrosarcoma cells expressing membrane-bound forms of IL-2 enhance antitumor immunity. J Microbiol and Biotech. 2006. 16:1919–1927.58. Chang MR, Lee WH, Choi JW, Park SO, Paik SG, Kim YS. Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2. Exp Mol Med. 2005. 37:240–249.
Article59. Choi JW, Lim HY, Chang M-R, Cheon J-Y, Kim YS. Anti-tumor immunity induced by tumor cells expresing a membrane-bound form of IL-2 and SDF-1. Animal Cells and systems. 2008. 12:193–201.
Article60. Tepper RI, Pattengale PK, Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989. 57:503–512.
Article61. Huang LR, Chen FL, Chen YT, Lin YM, Kung JT. Potent induction of long-term CD8+ T cell memory by short-term IL-4 exposure during T cell receptor stimulation. Proc Natl Acad Sci U S A. 2000. 97:3406–3411.
Article62. Schüler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8(+) T cells. J Exp Med. 2001. 194:1767–1775.
Article63. Schüler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med. 1999. 189:803–810.
Article64. Song K, Chang Y, Prud'homme GJ. Regulation of T-helper-1 versus T-helper-2 activity and enhancement of tumor immunity by combined DNA-based vaccination and non-viral cytokine gene transfer. Gene Ther. 2000. 7:481–492.
Article65. Chakrabarti R, Chang Y, Song K, Prud'homme GJ. Plasmids encoding membrane-bound IL-4 or IL-12 strongly costimulate DNA vaccination against carcinoembryonic antigen (CEA). Vaccine. 2004. 22:1199–1205.
Article66. Kim YS, Sonn CH, Paik SG, Bothwell AL. Tumor cells expressing membrane-bound form of IL-4 induce antitumor immunity. Gene Ther. 2000. 7:837–843.
Article67. Herbert AS, Heffron L, Sundick R, Roberts PC. Incorporation of membrane-bound, mammalian-derived immunomodulatory proteins into influenza whole virus vaccines boosts immunogenicity and protection against lethal challenge. Virol J. 2009. 6:42.
Article68. Stern AS, Podlaski FJ, Hulmes JD, Pan YC, Quinn PM, Wolitzky AG, Familletti PC, Stremlo DL, Truitt T, Chizzonite R, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990. 87:6808–6812.
Article69. Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989. 170:827–845.
Article70. Chan SH, Kobayashi M, Santoli D, Perussia B, Trinchieri G. Mechanisms of IFN-gamma induction by natural killer cell stimulatory factor (NKSF/IL-12). Role of transcription and mRNA stability in the synergistic interaction between NKSF and IL-2. J Immunol. 1992. 148:92–98.71. Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994. 153:1697–1706.72. Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992. 89:6065–6069.
Article73. Fan X, Sibalic V, Niederer E, Wüthrich RP. The proinflammatory cytokine interleukin-12 occurs as a cell membrane-bound form on macrophages. Biochem Biophys Res Commun. 1996. 225:1063–1067.
Article74. Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993. 178:1223–1230.
Article75. Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999. 27:58–63.
Article76. Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, Mier JW, Canning CM, Battiato L, Tahara H, Sherman ML. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997. 3:409–417.77. Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997. 90:2541–2548.78. Sun Y, Jurgovsky K, Möller P, Alijagic S, Dorbic T, Georgieva J, Wittig B, Schadendorf D. Vaccination with IL-12 gene-modified autologous melanoma cells: preclinical results and a first clinical phase I study. Gene Ther. 1998. 5:481–490.
Article79. Liu Y, Ehtesham M, Samoto K, Wheeler CJ, Thompson RC, Villarreal LP, Black KL, Yu JS. In situ adenoviral interleukin 12 gene transfer confers potent and long-lasting cytotoxic immunity in glioma. Cancer Gene Ther. 2002. 9:9–15.
Article80. Okada Y, Okada N, Mizuguchi H, Takahashi K, Hayakawa T, Mayumi T, Mizuno N. Optimization of antitumor efficacy and safety of in vivo cytokine gene therapy using RGD fiber-mutant adenovirus vector for preexisting murine melanoma. Biochim Biophys Acta. 2004. 1670:172–180.
Article81. Kang WK, Park C, Yoon HL, Kim WS, Yoon SS, Lee MH, Park K, Kim K, Jeong HS, Kim JA, Nam SJ, Yang JH, Son YI, Baek CH, Han J, Ree HJ, Lee ES, Kim SH, Kim DW, Ahn YC, Huh SJ, Choe YH, Lee JH, Park MH, Kong GS, Park EY, Kang YK, Bang YJ, Paik NS, Lee SN, Kim SH, Kim S, Robbins PD, Tahara H, Lotze MT, Park CH. Interleukin 12 gene therapy of cancer by peritumoral injection of transduced autologous fibroblasts: outcome of a phase I study. Hum Gene Ther. 2001. 12:671–684.
Article82. Cimino AM, Palaniswami P, Kim AC, Selvaraj P. Cancer vaccine development: protein transfer of membrane-anchored cytokines and immunostimulatory molecules. Immunol Res. 2004. 29:231–240.
Article83. Nagarajan S, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res. 2002. 62:2869–2874.84. Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996. 184:1953–1962.
Article85. Shaw SG, Maung AA, Steptoe RJ, Thomson AW, Vujanovic NL. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998. 161:2817–2824.86. Chen K, Braun S, Lyman S, Fan Y, Traycoff CM, Wiebke EA, Gaddy J, Sledge G, Broxmeyer HE, Cornetta K. Antitumor activity and immunotherapeutic properties of Flt3-ligand in a murine breast cancer model. Cancer Res. 1997. 57:3511–3516.87. Alsheikhly AR, Zweiri J, Walmesley AJ, Watson AJ, Christmas SE. Both soluble and membrane-bound forms of Flt3 ligand enhance tumor immunity following "suicide" gene therapy in a murine colon carcinoma model. Cancer Immunol Immunother. 2004. 53:946–954.
Article88. Wang YC, Zhu L, McHugh R, Sell KW, Selvaraj P. Expression of heat-stable antigen on tumor cells provides co-stimulation for tumor-specific T cell proliferation and cytotoxicity in mice. Eur J Immunol. 1995. 25:1163–1167.
Article89. Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem. 2001. 276:37993–38001.
Article90. Zhang X, Wei H, Chen Q, Tian Z. Activation of human natural killer cells by recombinant membrane-expressed fractalkine on the surface of tumor cells. Oncol Rep. 2007. 17:1371–1375.
Article91. Ren T, Chen Q, Tian Z, Wei H. Down-regulation of surface fractalkine by RNA interference in B16 melanoma reduced tumor growth in mice. Biochem Biophys Res Commun. 2007. 364:978–984.
Article92. Guo J, Zhang M, Wang B, Yuan Z, Guo Z, Chen T, Yu Y, Qin Z, Cao X. Fractalkine transgene induces T-cell-dependent antitumor immunity through chemoattraction and activation of dendritic cells. Int J Cancer. 2003. 103:212–220.
Article93. Tang L, Hu HD, Hu P, Lan YH, Peng ML, Chen M, Ren H. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007. 14:1226–1234.
Article94. Vitale S, Cambien B, Karimdjee BF, Barthel R, Staccini P, Luci C, Breittmayer V, Anjuère F, Schmid-Alliana A, Schmid-Antomarchi H. Tissue-specific differential antitumour effect of molecular forms of fractalkine in a mouse model of metastatic colon cancer. Gut. 2007. 56:365–372.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membrane-bound p35 Subunit of IL-12 on Tumor Cells is Functionally Equivalent to Membrane-bound Heterodimeric Single Chain IL-12 for Induction of Anti-tumor Immunity
- Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
- Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
- Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases
- Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2