Immune Netw.
2016 Oct;16(5):305-310. 10.4110/in.2016.16.5.305.
Membrane-bound p35 Subunit of IL-12 on Tumor Cells is Functionally Equivalent to Membrane-bound Heterodimeric Single Chain IL-12 for Induction of Anti-tumor Immunity
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon 34134, Korea. Young@cnu.ac.kr
- 2Institute of Biotechnology, Chungnam National University, Daejeon 34134, Korea. hlee@cnu.ac.kr
- KMID: 2355024
- DOI: http://doi.org/10.4110/in.2016.16.5.305
Abstract
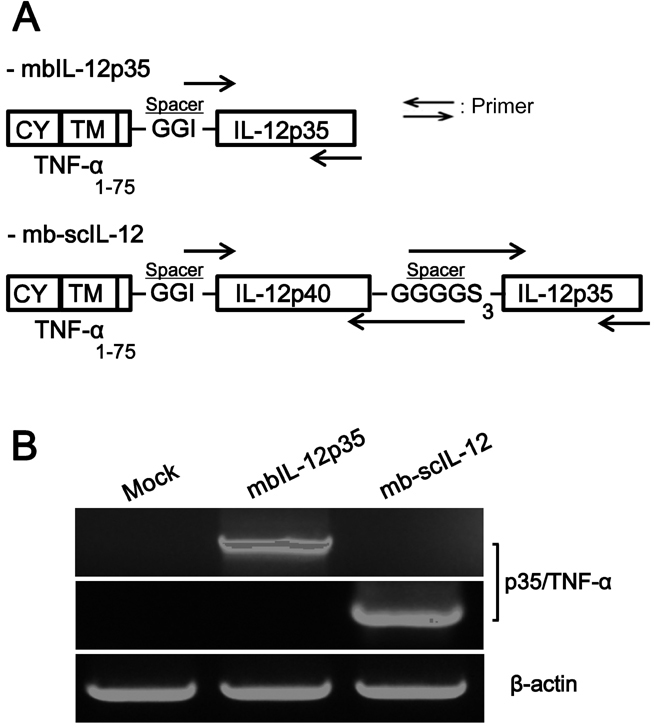
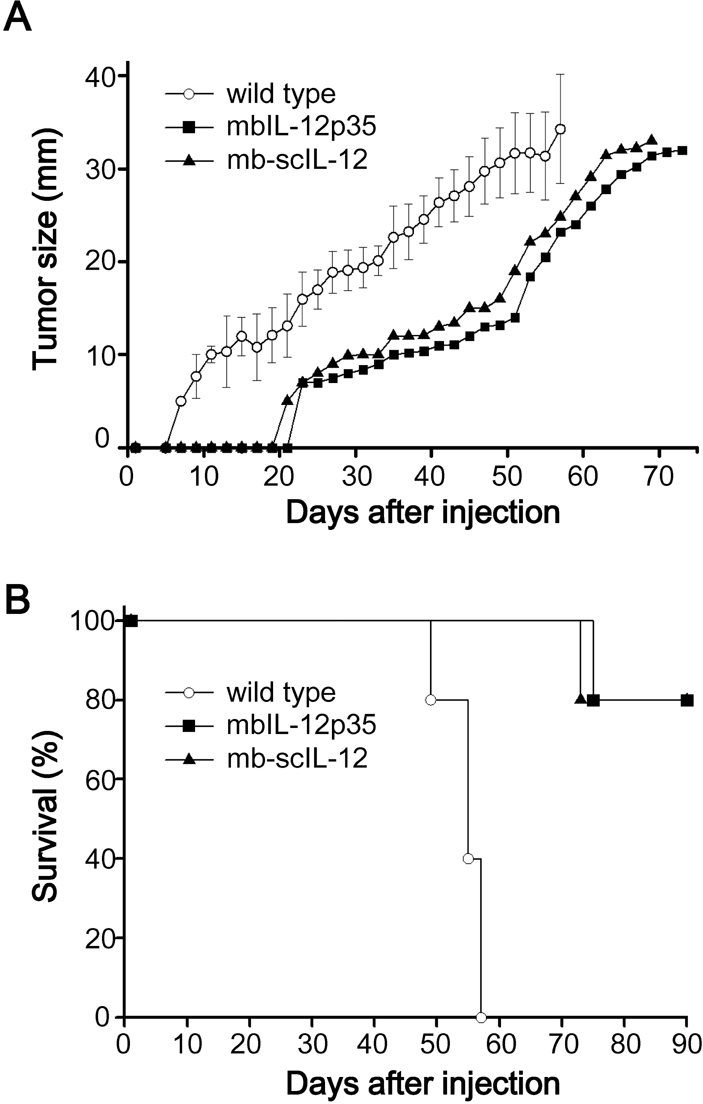
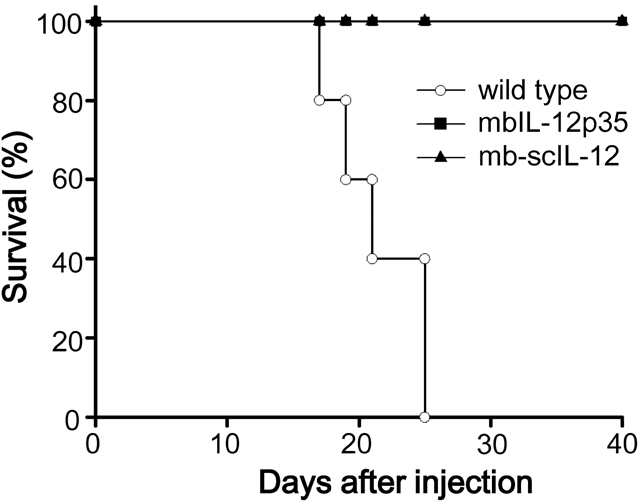
- In this study, we compared two different tumor cell vaccines for their induction of anti-tumor immunity; one was a tumor cell clone expressing a membrane-bound form of IL-12 p35 subunit (mbIL-12 p35 tumor clone), and the other was a tumor clone expressing heterodimeric IL-12 as a single chain (mb-scIL-12 tumor clone). The stimulatory effect of mb-scIL-12 on the proliferation of ConA-activated splenocytes was higher than that of mbIL-12 p35 in vitro. However, the stimulatory effect of mbIL-12 p35 was equivalent to that of recombinant soluble IL-12 (3 ng/ml). Interestingly, both tumor clones (mbIL-12 p35 and mb-scIL-12) showed similar tumorigenicity and induction of systemic anti-tumor immunity in vivo, suggesting that tumor cell expression of the membrane-bound p35 subunit is sufficient to induce anti-tumor immunity in our tumor vaccine model.
Figure
Cited by 2 articles
-
Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
Van Anh Do Thi, Sang Min Park, Hayyoung Lee, Young Sang Kim
Immune Netw. 2018;18(1):e12. doi: 10.4110/in.2018.18.e12.Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
Van Anh Do Thi, Sang Min Park, Hayyoung Lee, Young Sang Kim
Immune Netw. 2018;18(1):. doi: 10.4110/in.2018.18.e12.
Reference
-
1. Schoenhaut DS, Chua AO, Wolitzky AG, Quinn PM, Dwyer CM, McComas W, Familletti PC, Gately MK, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992; 148:3433–3440.2. Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994; 84:4008–4027.
Article3. Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993; 260:547–549.
Article4. Gately MK, Warrier RR, Honasoge S, Carvajal DM, Faherty DA, Connaughton SE, Anderson TD, Sarmiento U, Hubbard BR, Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994; 6:157–167.
Article5. Hendrzak JA, Brunda MJ. Antitumor and antimetastatic activity of interleukin-12. Curr Top Microbiol Immunol. 1996; 213(Pt 3):65–83.6. Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, Recombinant IL-12. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994; 153:1697–1706.7. Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993; 178:1223–1230.
Article8. Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997; 90:2541–2548.9. Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, Mier JW, Canning CM, Battiato L, Tahara H, Sherman ML. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997; 3:409–417.10. Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999; 27:58–63.
Article11. Mach N, Dranoff G. Cytokine-secreting tumor cell vaccines. Curr Opin Immunol. 2000; 12:571–575.
Article12. Chang CJ, Tai KF, Roffler S, Hwang LH. The immunization site of cytokine-secreting tumor cell vaccines influences the trafficking of tumor-specific T lymphocytes and antitumor efficacy against regional tumors. J Immunol. 2004; 173:6025–6032.
Article13. Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE, Wei Y. Synergistic anti-tumor effect of glycosylphosphatidylinositol-anchored IL-2 and IL-12. J Gene Med. 2004; 6:777–785.
Article14. Tahara H, Lotze MT. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995; 2:96–106.15. Kim YS, Sonn CH, Paik SG, Bothwell AL. Tumor cells expressing membrane-bound form of IL-4 induce antitumor immunity. Gene Ther. 2000; 7:837–843.
Article16. Chang MR, Lee WH, Choi JW, Park SO, Paik SG, Kim YS. Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2. Exp Mol Med. 2005; 37:240–249.
Article17. Lim HY, Ju HY, Chung HY, Kim YS. Antitumor effects of a tumor cell vaccine expressing a membrane-bound form of the IL-12 p35 subunit. Cancer Biol Ther. 2010; 10:336–343.
Article18. Lim H, Do SA, Park SM, Kim YS. Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit stimulates antitumor immune responses dominated by CD8(+) T Cells. Immune Netw. 2013; 13:63–69.
Article19. D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992; 176:1387–1398.20. Podlaski FJ, Nanduri VB, Hulmes JD, Pan YC, Levin W, Danho W, Chizzonite R, Gately MK, Stern AS. Molecular characterization of interleukin 12. Arch Biochem Biophys. 1992; 294:230–237.
Article21. Pan WY, Lo CH, Chen CC, Wu PY, Roffler SR, Shyue SK, Tao MH. Cancer immunotherapy using a membrane-bound interleukin-12 with B7-1 transmembrane and cytoplasmic domains. Mol Ther. 2012; 20:927–937.
Article22. Lode HN, Dreier T, Xiang R, Varki NM, Kang AS, Reisfeld RA. Gene therapy with a single chain interleukin 12 fusion protein induces T cell-dependent protective immunity in a syngeneic model of murine neuroblastoma. Proc Natl Acad Sci U S A. 1998; 95:2475–2480.
Article23. Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007; 450:566–569.
Article24. Wang Z, Liu JQ, Liu Z, Shen R, Zhang G, Xu J, Basu S, Feng Y, Bai XF. Tumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. . J Immunol. 2013; 190:2415–2423.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
- Tumor Therapy Applying Membrane-bound Form of Cytokines
- Induction of IL-12 Experession in Bone Marrow-derived Mouse Dendritic Cells
- Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
- IL-12 p40-Expressing Immune Cells Revealed by Cytokine Reporter Mouse System