Immune Netw.
2013 Apr;13(2):63-69. 10.4110/in.2013.13.2.63.
Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8+ T Cells
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon 305-764, Korea. young@cnu.ac.kr
- KMID: 1431927
- DOI: http://doi.org/10.4110/in.2013.13.2.63
Abstract
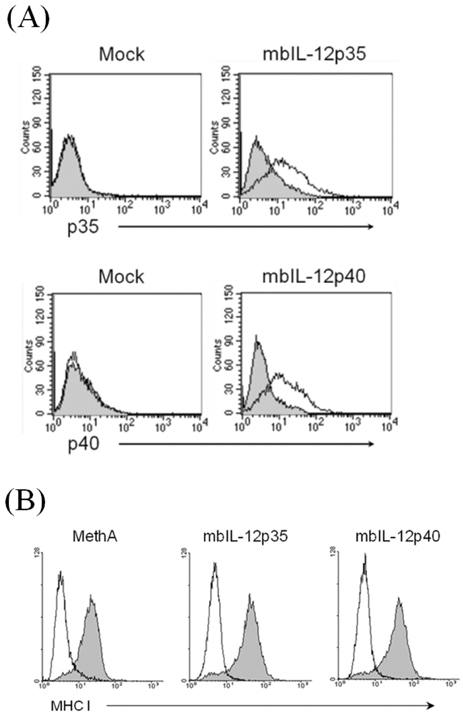
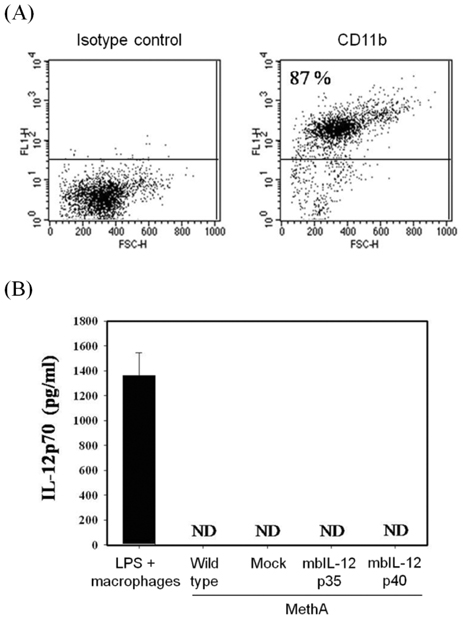
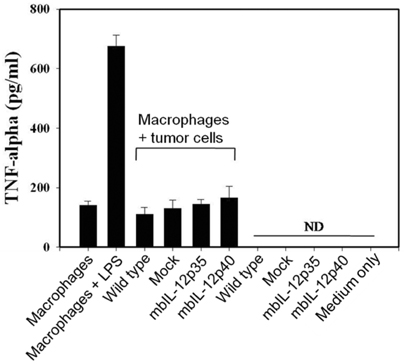
- IL-12 is a secretory heterodimeric cytokine composed of p35 and p40 subunits. IL-12 p35 and p40 subunits are sometimes produced as monomers or homodimers. IL-12 is also produced as a membrane-bound form in some cases. In this study, we hypothesized that the membrane-bound form of IL-12 subunits may function as a costimulatory signal for selective activation of TAA-specific CTL through direct priming without involving antigen presenting cells and helper T cells. MethA fibrosarcoma cells were transfected with expression vectors of membrane-bound form of IL-12p35 (mbIL-12p35) or IL-12p40 subunit (mbIL-12p40) and were selected under G418-containing medium. The tumor cell clones were analyzed for the expression of mbIL-12p35 or p40 subunit and for their stimulatory effects on macrophages. The responsible T-cell subpopulation for antitumor activity of mbIL-12p35 expressing tumor clone was also analyzed in T cell subset-depleted mice. Expression of transfected membrane-bound form of IL-12 subunits was stable during more than 3 months of in vitro culture, and the chimeric molecules were not released into culture supernatants. Neither the mbIL-12p35-expressing tumor clones nor mbIL-12p40-expressing tumor clones activated macrophages to secrete TNF-alpha. Growth of mbIL-12p35-expressing tumor clones was more accelerated in the CD8+ T cell-depleted mice than in CD4+ T cell-depleted or normal mice. These results suggest that CD8+ T cells could be responsible for the rejection of mbIL-12p35-expressing tumor clone, which may bypass activation of antigen presenting cells and CD4+ helper T cells.
MeSH Terms
-
Animals
Antigen-Presenting Cells
Clone Cells
Corynebacterium
Fibrosarcoma
Interleukin-12
Interleukin-12 Subunit p35
Interleukin-12 Subunit p40
Macrophages
Mice
Rejection (Psychology)
T-Lymphocytes
T-Lymphocytes, Helper-Inducer
Tumor Necrosis Factor-alpha
Corynebacterium
Interleukin-12
Interleukin-12 Subunit p35
Interleukin-12 Subunit p40
Tumor Necrosis Factor-alpha
Figure
Cited by 2 articles
-
The Nuclear Orphan Receptor NR4A1 is Involved in the Apoptotic Pathway Induced by LPS and Simvastatin in RAW 264.7 Macrophages
Yong Chan Kim, Seok Bean Song, Sang Kyu Lee, Sang Min Park, Young Sang Kim
Immune Netw. 2014;14(2):116-122. doi: 10.4110/in.2014.14.2.116.Membrane-bound p35 Subunit of IL-12 on Tumor Cells is Functionally Equivalent to Membrane-bound Heterodimeric Single Chain IL-12 for Induction of Anti-tumor Immunity
Hyun-Jin Kim, Sang Min Park, Hayyoung Lee, Young Sang Kim
Immune Netw. 2016;16(5):305-310. doi: 10.4110/in.2016.16.5.305.
Reference
-
1. Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989. 170:827–845.
Article2. Gately MK, Wilson DE, Wong HL. Synergy between recombinant interleukin 2 (rIL 2) and IL 2-depleted lymphokine-containing supernatants in facilitating allogeneic human cytolytic T lymphocyte responses in vitro. J Immunol. 1986. 136:1274–1282.3. Stern AS, Podlaski FJ, Hulmes JD, Pan YC, Quinn PM, Wolitzky AG, Familletti PC, Stremlo DL, Truitt T, Chizzonite R, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci U S A. 1990. 87:6808–6812.
Article4. Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993. 260:547–549.
Article5. Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993. 177:1199–1204.
Article6. Gately MK, Desai BB, Wolitzky AG, Quinn PM, Dwyer CM, Podlaski FJ, Familletti PC, Sinigaglia F, Chizonnite R, Gubler U, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol. 1991. 147:874–882.7. Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998. 70:83–243.
Article8. Weiss JM, Subleski JJ, Wigginton JM, Wiltrout RH. Immunotherapy of cancer by IL-12-based cytokine combinations. Expert Opin Biol Ther. 2007. 7:1705–1721.
Article9. D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992. 176:1387–1398.10. Gillessen S, Carvajal D, Ling P, Podlaski FJ, Stremlo DL, Familletti PC, Gubler U, Presky DH, Stern AS, Gately MK. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995. 25:200–206.
Article11. Heinzel FP, Hujer AM, Ahmed FN, Rerko RM. In vivo production and function of IL-12 p40 homodimers. J Immunol. 1997. 158:4381–4388.12. Ling P, Gately MK, Gubler U, Stern AS, Lin P, Hollfelder K, Su C, Pan YC, Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995. 154:116–127.13. Jana M, Dasgupta S, Saha RN, Liu X, Pahan K. Induction of tumor necrosis factor-alpha (TNF-alpha) by interleukin-12 p40 monomer and homodimer in microglia and macrophages. J Neurochem. 2003. 86:519–528.
Article14. Jana M, Pahan K. IL-12 p40 homodimer, but not IL-12 p70, induces the expression of IL-16 in microglia and macrophages. Mol Immunol. 2009. 46:773–783.
Article15. Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007. 28:33–38.
Article16. Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J Exp Med. 2006. 203:1805–1815.
Article17. Fan X, Sibalic V, Niederer E, Wüthrich RP. The proinflammatory cytokine interleukin-12 occurs as a cell membrane-bound form on macrophages. Biochem Biophys Res Commun. 1996. 225:1063–1067.
Article18. Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991. 146:3074–3081.19. Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993. 178:1223–1230.
Article20. Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994. 153:1697–1706.21. Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, Mier JW, Canning CM, Battiato L, Tahara H, Sherman ML. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997. 3:409–417.22. Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999. 27:58–63.
Article23. Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997. 90:2541–2548.24. Kim YS. Tumor Therapy Applying Membrane-bound Form of Cytokines. Immune Netw. 2009. 9:158–168.
Article25. Li Q, Li L, Shi W, Jiang X, Xu Y, Gong F, Zhou M, Edwards CK 3rd, Li Z. Mechanism of action differences in the antitumor effects of transmembrane and secretory tumor necrosis factor-alpha in vitro and in vivo. Cancer Immunol Immunother. 2006. 55:1470–1479.
Article26. Rieger R, Whitacre D, Cantwell MJ, Prussak C, Kipps TJ. Chimeric form of tumor necrosis factor-alpha has enhanced surface expression and antitumor activity. Cancer Gene Ther. 2009. 16:53–64.
Article27. el-Shami KM, Tzehoval E, Vadai E, Feldman M, Eisenbach L. Induction of antitumor immunity with modified autologous cells expressing membrane-bound murine cytokines. J Interferon Cytokine Res. 1999. 19:1391–1401.
Article28. Soo Hoo W, Lundeen KA, Kohrumel JR, Pham NL, Brostoff SW, Bartholomew RM, Carlo DJ. Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J Immunol. 1999. 162:7343–7349.29. Yei S, Bartholomew RM, Pezzoli P, Gutierrez A, Gouveia E, Bassett D, Soo Hoo W, Carlo DJ. Novel membrane-bound GM-CSF vaccines for the treatment of cancer: generation and evaluation of mbGM-CSF mouse B16F10 melanoma cell vaccine. Gene Ther. 2002. 9:1302–1311.
Article30. Chang MR, Lee WH, Choi JW, Park SO, Paik SG, Kim YS. Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2. Exp Mol Med. 2005. 37:240–249.
Article31. Choi JW, Lim HY, Chang MR, Cheon JY, Kim YS. Anti-tumor immunity induced by tumor cells expresing a membrane-bound form of IL-2 and SDF-1. Animal Cells Syst. 2008. 12:193–201.
Article32. Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE, Wei Y. Glycoinositol phospholipid-anchored interleukin 2 but not secreted interleukin 2 inhibits melanoma tumor growth in mice. Mol Cancer Ther. 2002. 1:1019–1024.33. Ji J, Li J, Holmes LM, Burgin KE, Yu X, Wagner TE, Wei Y. Synergistic anti-tumor effect of glycosylphosphatidylinositol-anchored IL-2 and IL-12. J Gene Med. 2004. 6:777–785.
Article34. Sonn CH, Yoon HR, Seong IO, Chang MR, Kim YC, Kang HC, Suh SC, Kim YS. MethA Fibrosarcoma Cells Expressing Membrane-Bound Forms of IL-2 Enhance Antitumor Immunity. Korean J Microbiol Biotechnol. 2006. 16:1919–1927.35. Chakrabarti R, Chang Y, Song K, Prud'homme GJ. Plasmids encoding membrane-bound IL-4 or IL-12 strongly costimulate DNA vaccination against carcinoembryonic antigen (CEA). Vaccine. 2004. 22:1199–1205.
Article36. Kim YS, Sonn CH, Paik SG, Bothwell AL. Tumor cells expressing membrane-bound form of IL-4 induce antitumor immunity. Gene Ther. 2000. 7:837–843.
Article37. Cimino AM, Palaniswami P, Kim AC, Selvaraj P. Cancer vaccine development: protein transfer of membrane-anchored cytokines and immunostimulatory molecules. Immunol Res. 2004. 29:231–240.
Article38. Lim HY, Ju HY, Chung HY, Kim YS. Antitumor effects of a tumor cell vaccine expressing a membrane-bound form of the IL-12 p35 subunit. Cancer Biol Ther. 2010. 10:336–343.
Article39. Baek S, Lee SJ, Kim MJ, Lee H. Dendritic Cell (DC) Vaccine in Mouse Lung Cancer Minimal Residual Model; Comparison of Monocyte-derived DC vs. Hematopoietic Stem Cell Derived-DC. Immune Netw. 2012. 12:269–276.
Article40. Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995. 270:908.
Article41. Okada Y, Okada N, Mizuguchi H, Takahashi K, Hayakawa T, Mayumi T, Mizuno N. Optimization of antitumor efficacy and safety of in vivo cytokine gene therapy using RGD fiber-mutant adenovirus vector for preexisting murine melanoma. Biochim Biophys Acta. 2004. 1670:172–180.
Article42. Sun Y, Jurgovsky K, Möller P, Alijagic S, Dorbic T, Georgieva J, Wittig B, Schadendorf D. Vaccination with IL-12 gene-modified autologous melanoma cells: preclinical results and a first clinical phase I study. Gene Ther. 1998. 5:481–490.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membrane-bound p35 Subunit of IL-12 on Tumor Cells is Functionally Equivalent to Membrane-bound Heterodimeric Single Chain IL-12 for Induction of Anti-tumor Immunity
- Tumor Therapy Applying Membrane-bound Form of Cytokines
- Antitumor immunity induced by tumor cells engineered to express a membrane-bound form of IL-2
- Ectopically Expressed Membrane-bound Form of IL-9 Exerts Immune-stimulatory Effect on CT26 Colon Carcinoma Cells
- Induction of Unique STAT Heterodimers by IL-21 Provokes IL-1RI Expression on CD8 + T Cells, Resulting in Enhanced IL-1β Dependent Effector Function