J Bacteriol Virol.
2011 Mar;41(1):47-54. 10.4167/jbv.2011.41.1.47.
Molecular Identification of the Vaccine Strain from the Inactivated Rabies Vaccine
- Affiliations
-
- 1National Veterinary Research and Quarantine Service, Anyang, Korea, MIFAFF, Korea. yangdk@korea.kr
- KMID: 1449862
- DOI: http://doi.org/10.4167/jbv.2011.41.1.47
Abstract
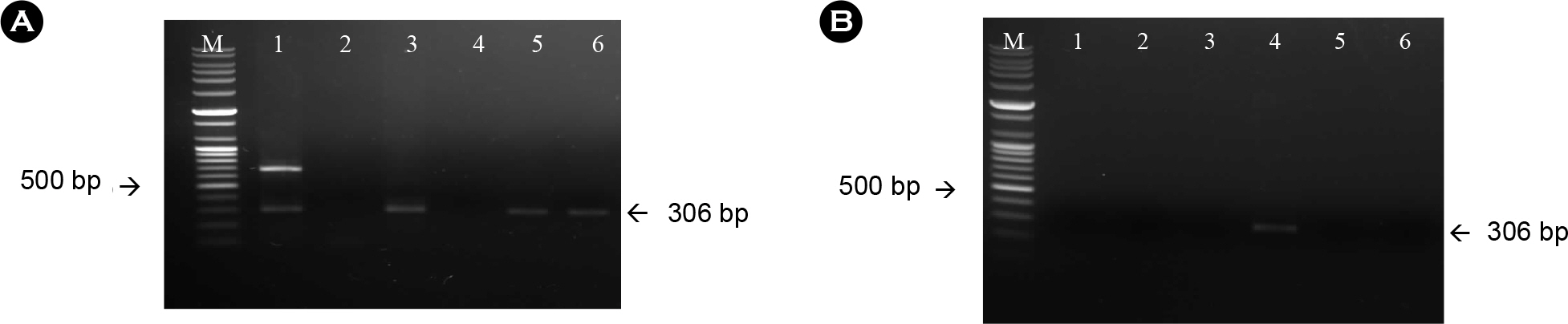
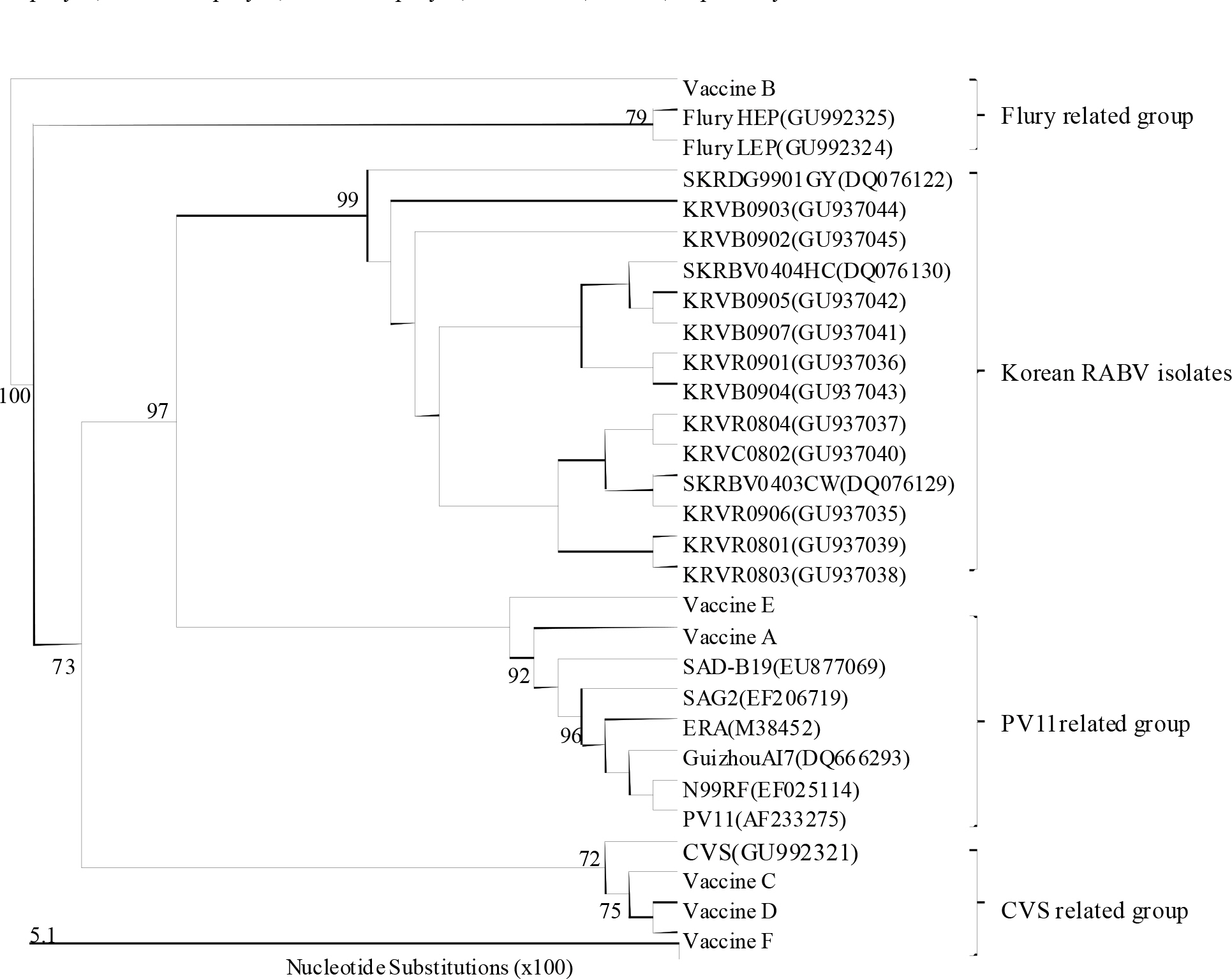
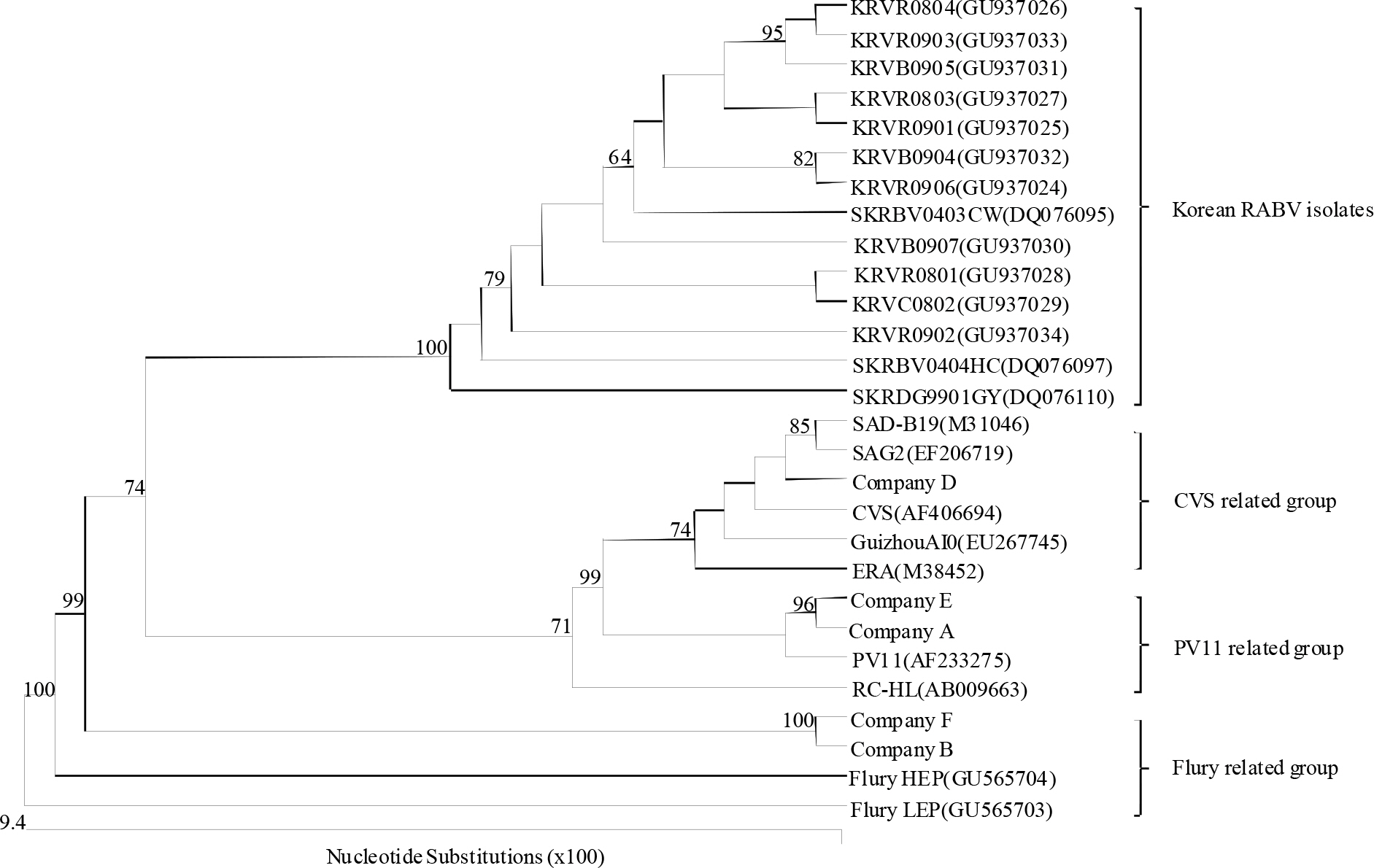
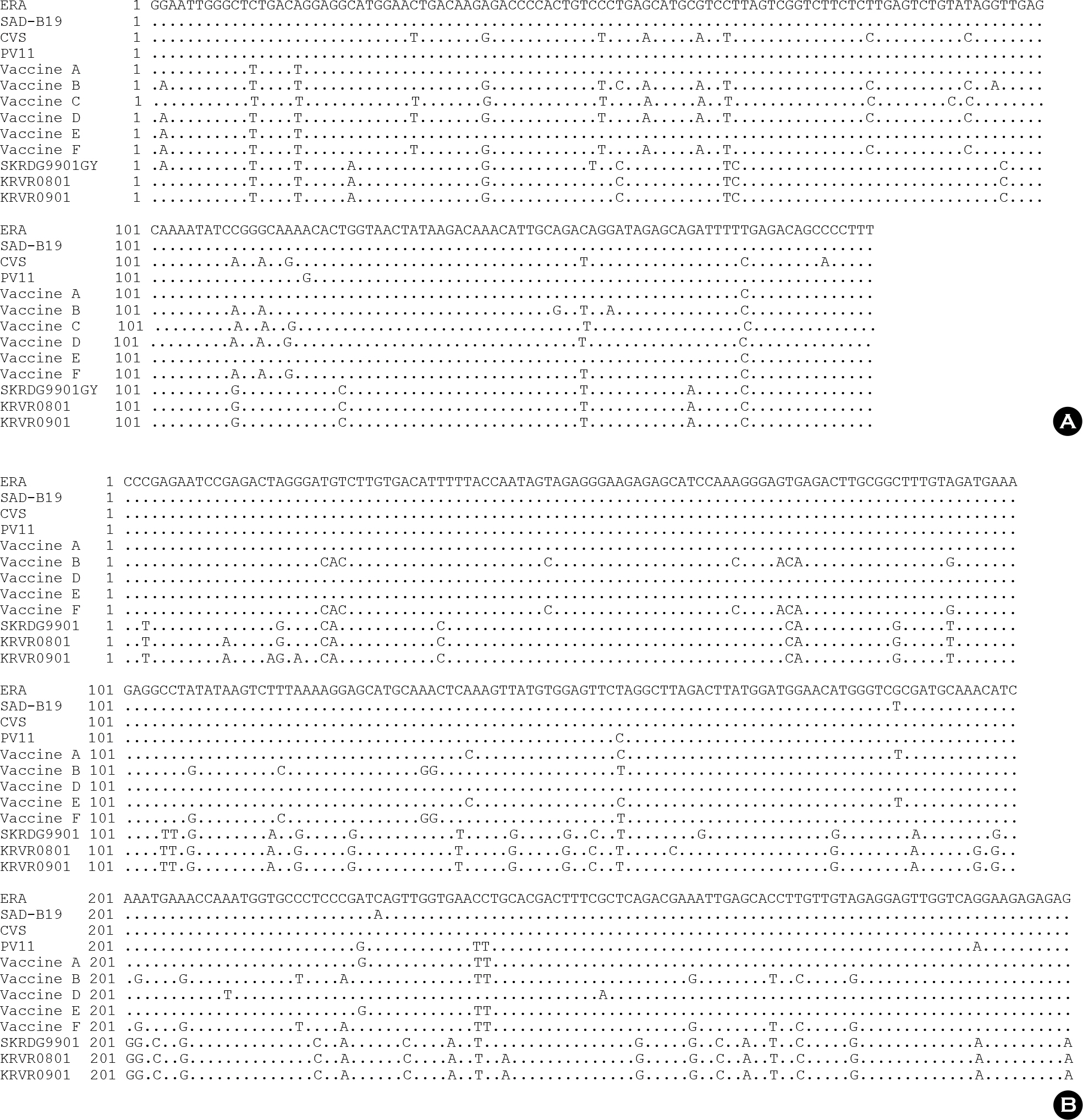
- Since 1994, several different inactivated rabies vaccines have been used to immunize domestic animals such as dogs, cats, and cattle in South Korea. The Korean Veterinary Authority has conducted safety and efficacy testes of inactivated vaccines using laboratory animals. In this study, we applied a molecular method to investigate the genetic characterization of the rabies virus (RABV) genes in six commercial inactivated rabies vaccines, and determined the efficiency of two extraction reagents (i.e., sodium citrate or isopropyl myristate) to separate the vaccine antigens from the antigen/adjuvant complexes. Six partial nucleocapsid (N: 181 bp) and five partial glycoprotein (G: 306 bp) genes were successfully amplified with specific primer sets, which demonstrated that sodium citrate is more efficient than isopropyl myristate in extracting viral RNA from inactivated gel vaccines. In addition, we identified the viral strain of the vaccine by analyzing the nucleotide sequences of the N and the G genes. The nucleotide similarity of the partial N and G genes ranged from 97.1 to 99.4% and from 91.8 to 100% among rabies vaccine strains, respectively, indicating that each manufacturer used different rabies virus strains to produce their vaccines. The molecular method used in this study could also be used to identify viral strains in other inactivated vaccines.
MeSH Terms
-
Animals
Animals, Domestic
Animals, Laboratory
Base Sequence
Cats
Cattle
Citrates
Citric Acid
Dogs
Glycoproteins
Indicators and Reagents
Myristates
Myristic Acid
Nucleocapsid
Rabies
Rabies Vaccines
Rabies virus
Republic of Korea
RNA, Viral
Sodium
Sprains and Strains
Testis
Vaccines
Vaccines, Inactivated
Citrates
Citric Acid
Glycoproteins
Indicators and Reagents
Myristates
Myristic Acid
RNA, Viral
Rabies Vaccines
Sodium
Vaccines
Vaccines, Inactivated
Figure
Cited by 3 articles
-
The present and future of rabies vaccine in animals
Dong-Kun Yang, Ha-Hyun Kim, Kyung-Woo Lee, Jae-Young Song
Clin Exp Vaccine Res. 2013;2(1):19-25. doi: 10.7774/cevr.2013.2.1.19.A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice
Dong-Kun Yang, Keisuke Nakagawa, Naoto Ito, Ha-Hyun Kim, Bang-Hun Hyun, Jin-Ju Nah, Makoto Sugiyama, Jae-Young Song
Clin Exp Vaccine Res. 2014;3(2):176-184. doi: 10.7774/cevr.2014.3.2.176.Antibody Response in Cattle and Guinea Pigs Inoculated with Rabies Vaccines
Dong-Kun Yang, Woong-Ho Jeong, Ha-Hyun Kim, Jin-Ju Nah, Jae-Jo Kim, Sung-Suk Choi, Jong-Taek Kim, Jae-Young Song
J Bacteriol Virol. 2014;44(1):67-74. doi: 10.4167/jbv.2014.44.1.67.
Reference
-
1). Tordo N., Marianneau MP. Viruses and bats: rabies and Lyssavirus. Bull Mem Acad R Med Belg. 2009. 164:7–15.2). World Health Organization (WHO). WHO Expert Committee on Rabies. World Health Organ Tech Rep Ser. 1992. 824:1–84.3). Hyun BH., Lee KK., Kim IJ., Lee KW., Park HJ., Lee OS, et al. Molecular epidemiology of rabies virus isolates from South Korea. Virus Res. 2005. 114:113–25.
Article4). Kim CH., Lee CG., Yoon HC., Nam HM., Park CK., Lee JC, et al. Rabies, an emerging disease in Korea. J Vet Med B Infect Dis Vet Public Health. 2006. 53:111–5.
Article5). Park YJ., Shin MK., Kwon HM. Genetic characterization of rabies virus isolates in Korea. Virus Genes. 2005. 30:341–7.
Article6). Kwon YB., Kim YH., Lim YM. Studies on the production of rabies live vaccine. I. biological properties of the experimentally produced tissue culture attenuated live vaccine. Res Rep ORD. 1981. 23:125–35.7). Hwang EK. Outbreak and control of rabies in animals in Korea. Korean J Vet Public Health. 1995. 19:281–93.8). Lee JH., Lee MJ., Lee JB., Kim JS., Bae CS., Lee WC. Review of canine rabies prevalence under two different vaccination programmes in Korea. Vet Rec. 2001. 148:511–2.
Article9). Dreesen DW. Animal vaccine. Jackson AC & Wunner WH, editor. editors.Rabies. 2nd ed.London: Academic Press;2007. p. 517–27.10). Animal and plant health inspection service (APHIS), Department of agriculture. 9 Code of Federal Regulations. 1-1-10th. Washington D. C: US government Printing Office. 2010. 741–3.11). European pharmacopoeia. Strasbourg, European Directorate for the Quality of Medicines & Healthcare. 6th ed. 2007. 836–8.12). Süliová J., Benísek Z., Svrcek S., Durove A., Ondrejka R. The effectiveness of inactivated, purified and concentrated experimental rabies vaccine for veterinary use: immunogenic activity. Vet Med (Praha). 1997. 42:51–6.13). Yang J., Hooper DC., Wunner WH., Koprowski H., Dietzschold B., Fu ZF. The specificity of rabies virus RNA encapsidation by nucleoprotein. Virology. 1998. 242:107–17.
Article14). Hooper DC., Sauder C., Scott GS., Dietzschold B., Richt JA. Immunopathology and immunoprotection in CNS virus infections: mechanisms of virus clearance from the CNS. Curr Top Microbiol Immunol. 2002. 265:163–82.
Article15). Guyatt KJ., Twin J., Davis P., Holmes EC., Smith GA., Smith IL, et al. A molecular epidemiological study of Australian bat lyssavirus. J Gen Virol. 2003. 84:485–96.
Article16). Morimoto K., Hooper DC., Spitsin S., Koprowski H., Dietzschold B. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J Virol. 1999. 73:510–8.
Article17). Choi JG., Lee YJ., Kim JY., Kim YH., Paek MR., Yang DK, et al. Molecular identification of the vaccine strain from the inactivated oil emulsion H9N2 low pathogenic avian influenza vaccine. J Vet Sci. 2010. 11:161–3.
Article18). Maas R., van Diepen M., Komen M., Oei H., Claassen I. Antigen content of inactivated Newcastle disease oil emulsion vaccines as an in vitro indicator of potency. Dev Biol (Basel). 2002. 111:313–8.19). Steinhauer DA., Holland JJ. Rapid evolution of RNA viruses. Annu Rev Microbiol. 1987. 41:409–33.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunogenicity of an inactivated rabies vaccine for animals derived from the recombinant ERAGS strain
- The present and future of rabies vaccine in animals
- Molecular identification of the vaccine strain from the inactivated oil emulsion H9N2 low pathogenic avian influenza vaccine
- Antibody Response in Korean Raccoon Dogs Inoculated with Inactivated Rabies Vaccines
- Strategies to maintain Korea's animal rabies non-occurrence status