J Bacteriol Virol.
2012 Sep;42(3):242-246. 10.4167/jbv.2012.42.3.242.
Antibody Response in Korean Raccoon Dogs Inoculated with Inactivated Rabies Vaccines
- Affiliations
-
- 1Animal, Plant and Fisheries Quarantine and Inspection Agency, Anyang, Gyeonggi-do; MIFAFF, Republic of Korea. yangdk@korea.kr
- 2Wild Life Center, Gyeonggi-do Veterinary Service Laboratory, Korea.
- KMID: 1434753
- DOI: http://doi.org/10.4167/jbv.2012.42.3.242
Abstract
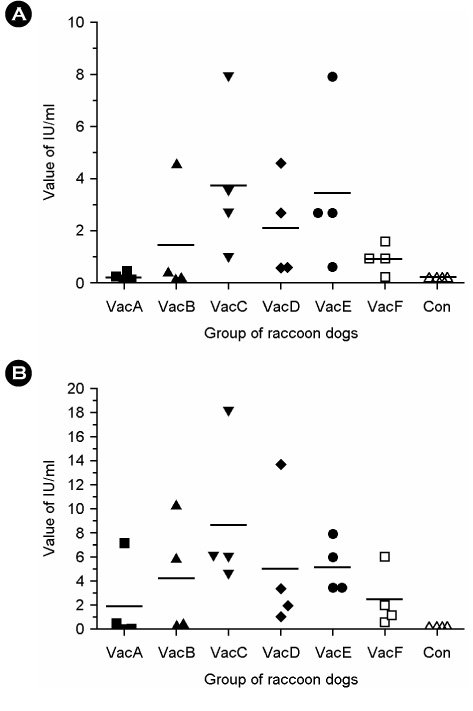
- Since sylvatic rabies was first identified in South Korea in 1993, over three million bait vaccine doses have been distributed to rabies risk regions in order to block transmission of rabies among wild animals. New progressive strategy is needed to eliminate sylvatic rabies completely in rabies risk regions. Before applying the preventive program related to eradication, immunogenicity of inactivated rabies vaccines available in Korea has to be evaluated in Korean raccoon dogs (Nyctereutes procyonoides koreensis). Six groups of raccoon dogs in wild rescue center of Gyeonggi-do were vaccinated intramuscularly with single dose of six inactivated commercial rabies vaccines (designated A to F). Serum samples at the time of vaccination, and two and four weeks post vaccination were obtained and analyzed by virus neutralizing assay (VNA). All raccoon dogs inoculated with vaccines C, D, E or F, showed VN antibody titers ranging from 0.5 to 13.77 IU/ml. Half of four raccoon dogs immunized with vaccine B revealed VN titer over 0.5 IU/ml, and one of four raccoon dogs inoculated with vaccine A showed protective antibody titer. This finding suggests that most of the commercially available inactivated rabies vaccines could induce protective immunity in Korean raccoon dogs and be applicable to new rabies control program.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
Dong-Kun Yang, Ha-Hyun Kim, Sung-Suk Choi, Jong-Tack Kim, Kang-Bok Lee, Seong Heon Lee, In-Soo Cho
Clin Exp Vaccine Res. 2016;5(2):159-168. doi: 10.7774/cevr.2016.5.2.159.Serologic Survey of Rabies Virus, Canine Distemper Virus and Parvovirus in Wild Raccoon Dogs (
Nyctereutes procyonoides koreensis ) in Korea
Dong-Kun Yang, Ha-Hyun Kim, Jin-Ju Nah, Sung-Suk Choi, Jong-Taek Kim, Woong-Ho Jeong, Jae-Young Song
J Bacteriol Virol. 2013;43(3):204-209. doi: 10.4167/jbv.2013.43.3.204.
Reference
-
1. WHO expert committee on rabies. World Health Organ Tech Rep Ser. 1992. 824:1–84.2. Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011. 64:513–515.
Article3. Kim CH, Lee CG, Yoon HC, Nam HM, Park CK, Lee JC, et al. Rabies, an emerging disease in Korea. J Vet Med B Infect Dis Vet Public Health. 2006. 53:111–115.
Article4. Yang DK, Kim SY, Oh YI, Lee JA, Cho SD, Lee KW, et al. Epidemiological characteristics of rabies in South Korea from January 2004 to March 2011. J Bacteriol Virol. 2011. 41:165–171.
Article5. Johnson N, Black C, Smith J, Un H, McElhinney LM, Aylan O, et al. Rabies emergence among foxes in Turkey. J Wildl Dis. 2003. 39:262–270.
Article6. Kuzmin IV, Botvinkin AD, McElhinney LM, Smith JS, Orciari LA, Hughes GJ, et al. Molecular epidemiology of terrestrial rabies in the former Soviet Union. J Wildl Dis. 2004. 40:617–631.
Article7. Smith JS, Orciari LA, Yager PA, Seidel HD, Warner CK. Epidemiologic and historical relationships among 87 rabies virus isolates as determined by limited sequence analysis. J Infect Dis. 1992. 166:296–307.
Article8. Park YJ, Shin MK, Kwon HM. Genetic characterization of rabies virus isolates in Korea. Virus Genes. 2005. 30:341–347.
Article9. Brown CL, Rupprecht CE. Vaccination of free-ranging Pennsylvania raccoons (Procyon lotor) with inactivated rabies vaccine. J Wildl Dis. 1990. 26:253–257.
Article10. Rosatte RC, Howard DR, Campbell JB, MacInnes CD. Intramuscular vaccination of skunks and raccoons against rabies. J Wildl Dis. 1990. 26:225–230.
Article11. Sobey KG, Rosatte R, Bachmann P, Buchanan T, Bruce L, Donovan D, et al. Field evaluation of an inactivated vaccine to control raccoon rabies in Ontario, Canada. J Wildl Dis. 2010. 46:818–831.
Article12. Cliquet F, Aubert M, Sagné L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998. 212:79–87.
Article13. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al. Field virology. 2001. Philadelphia: Lippincott Williams & Wilkins;1221–1277.14. Fehlner-Gardiner C, Rudd R, Donovan D, Slate D, Kempf L, Badcock J. Comparing ONRAB® AND RABORAL V-RG® oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA. J Wildl Dis. 2012. 48:157–167.
Article15. Sterner RT, Meltzer MI, Shwiff SA, Slate D. Tactics and economics of wildlife oral rabies vaccination, Canada and the United States. Emerg Infect Dis. 2009. 15:1176–1184.
Article16. Lalosević D, Lalosević V, Lazarević-Ivanc LJ, Knezević I. BHK-21 cell culture rabies vaccine: immunogenicity of a candidate vaccine for humans. Dev Biol (Basel). 2008. 131:421–429.17. Aubert MF. Practical significance of rabies antibodies in cats and dogs. Rev Sci Tech. 1992. 11:735–760.
Article18. Hanlon CA, Niezgoda M, Hamir AN, Schumacher C, Koprowski H, Rupprecht CE. First North American field release of a vaccinia-rabies glycoprotein recombinant virus. J Wildl Dis. 1998. 34:228–239.
Article19. Minke JM, Bouvet J, Cliquet F, Wasniewski M, Guiot AL, Lemaitre L, et al. Comparison of antibody responses after vaccination with two inactivated rabies vaccines. Vet Microbiol. 2009. 133:283–286.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibody Response in Cattle and Guinea Pigs Inoculated with Rabies Vaccines
- Outbreaks and Control of Animal Rabies in Korea
- Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
- Safety and Immunogenicity of a Recombinant Rabies Virus Strain (ERAG3G) in Korean Raccoon Dogs
- Rabies immune status of raccoon dogs residing in areas where rabies bait vaccine has been distributed