Korean J Physiol Pharmacol.
2011 Jun;15(3):157-162. 10.4196/kjpp.2011.15.3.157.
Genipin Selectively Inhibits TNF-alpha-activated VCAM-1 But Not ICAM-1 Expression by Upregulation of PPAR-gamma in Human Endothelial Cells
- Affiliations
-
- 1Department of Urology, School of Medicine, and Institute of Health Sciences, Gyeongsang National University, Jinju 660-290, Korea.
- 2Department of Pharmacology, School of Medicine, and Institute of Health Sciences, Gyeongsang National University, Jinju 660-290, Korea. kcchang@gnu.kr
- 3College of Pharmacy, Sungkyunkwan University, Suwon 440-746, Korea.
- 4Division of Biosciences, Dongguk University, Gyeongju 780-714, Korea. dulee@dongguk.ac.kr
- KMID: 1448919
- DOI: http://doi.org/10.4196/kjpp.2011.15.3.157
Abstract
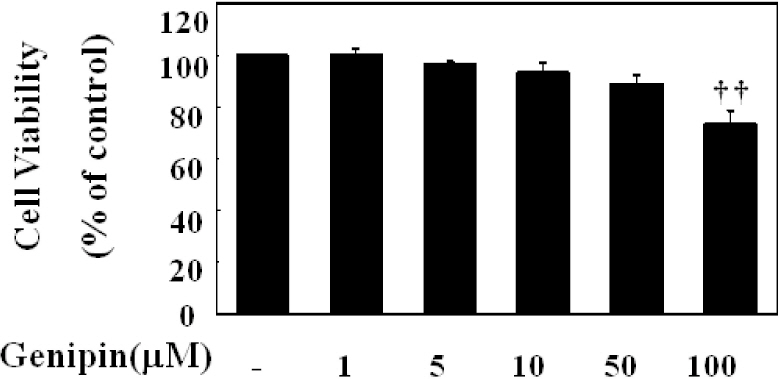
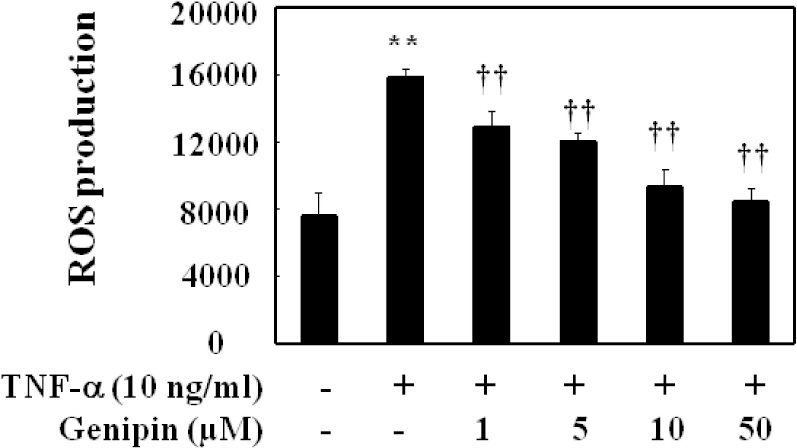
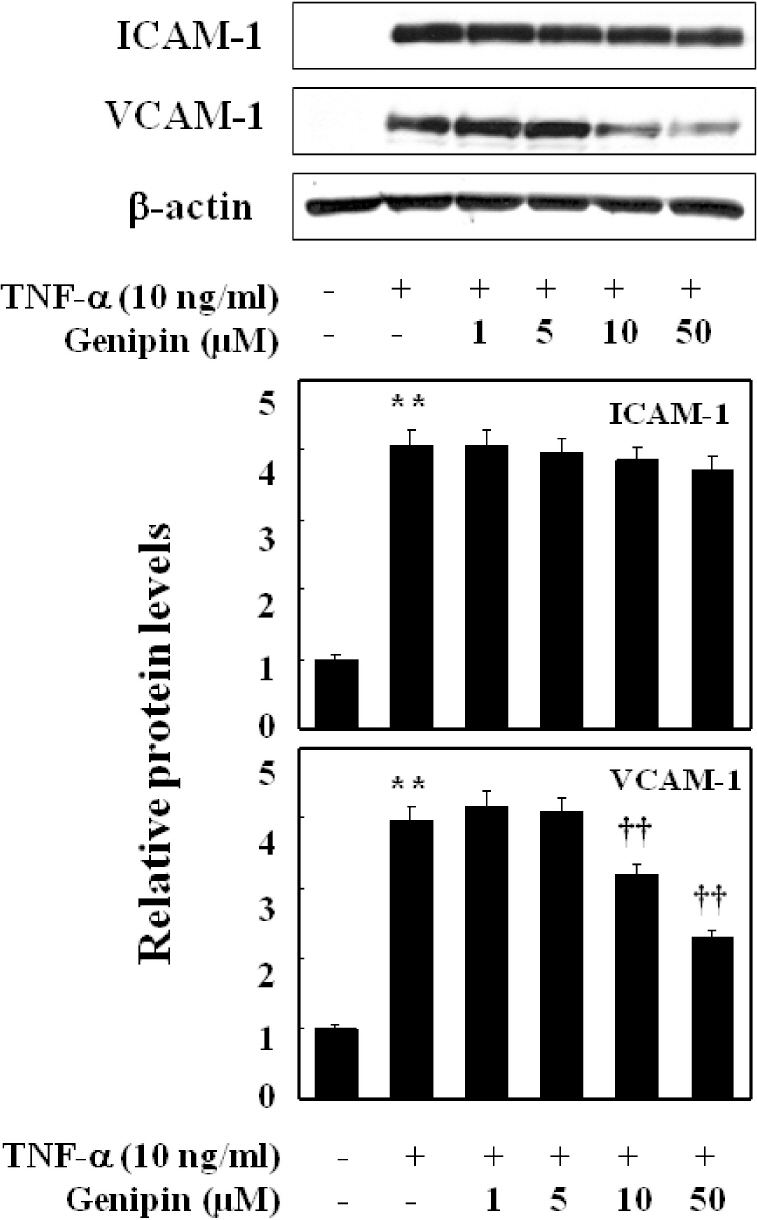
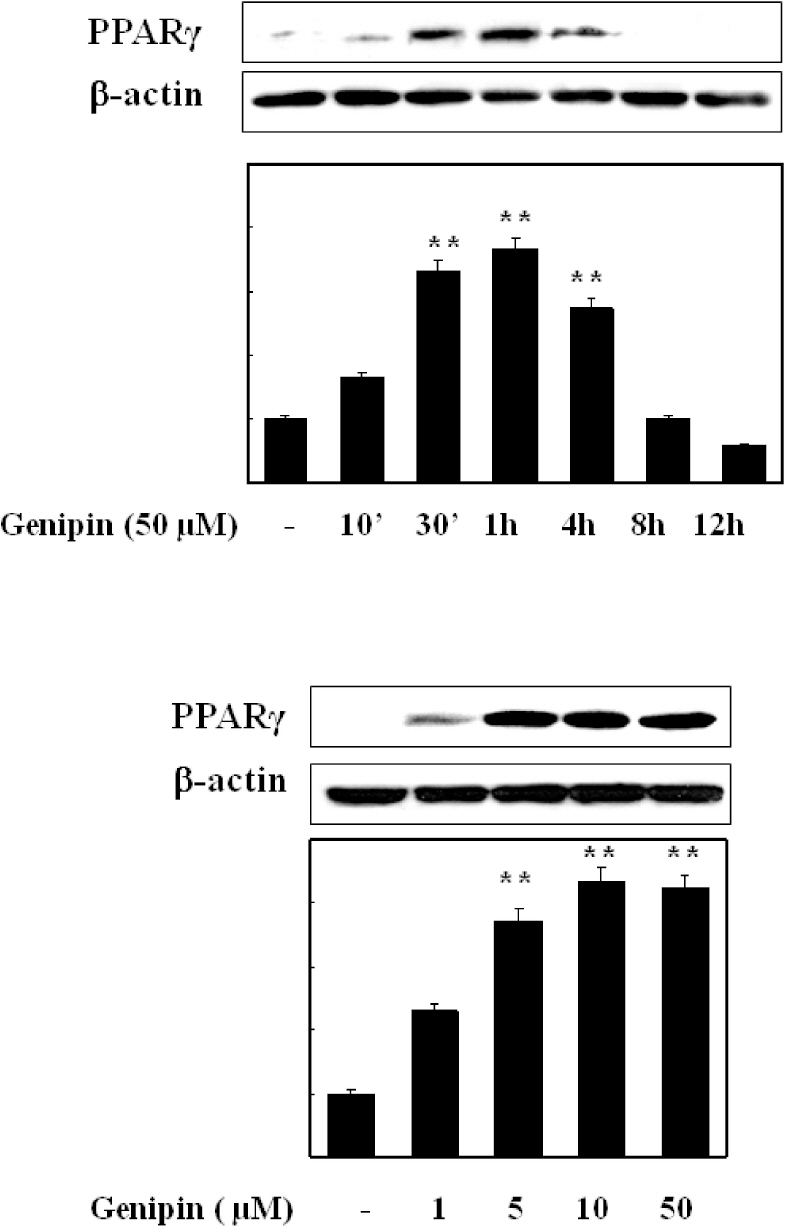
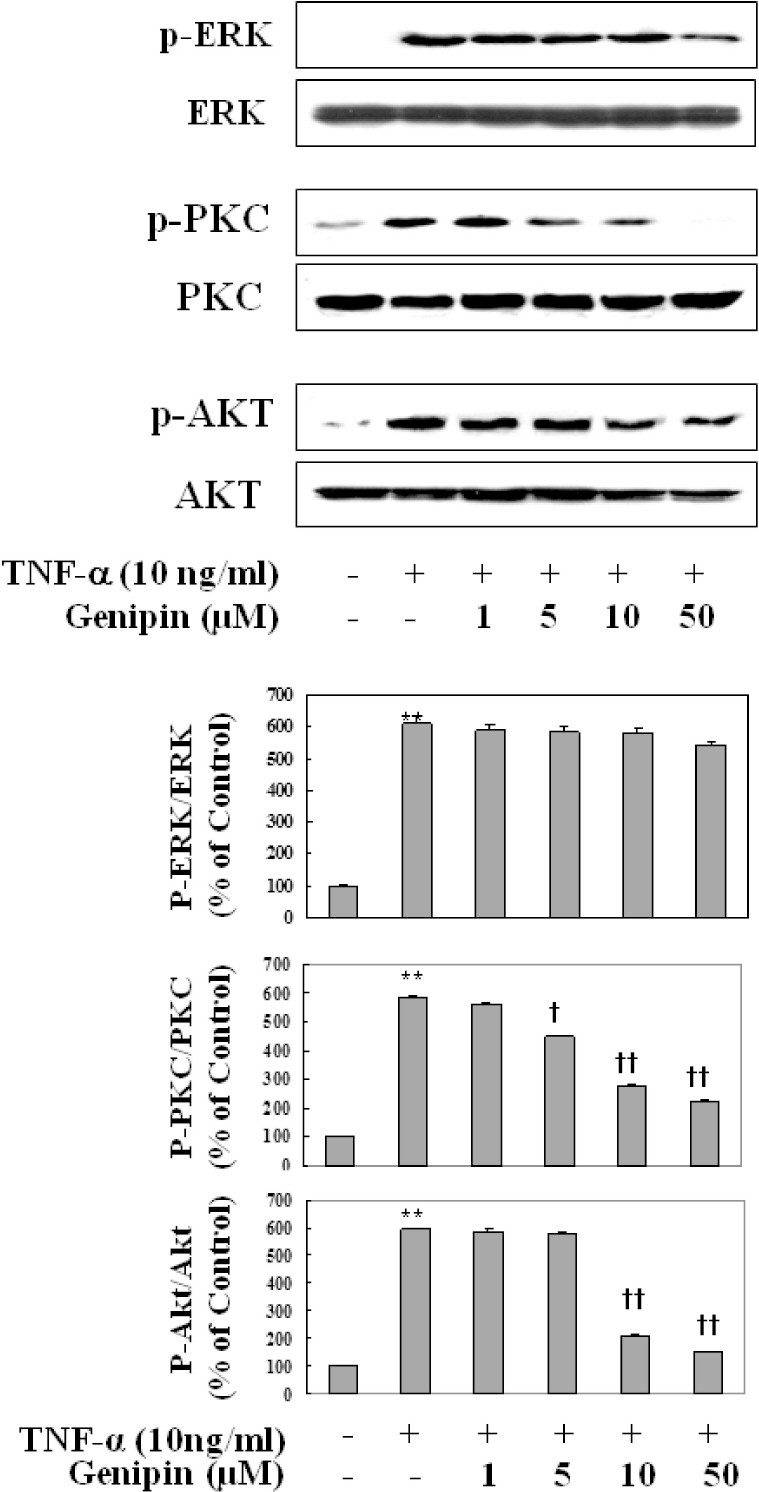
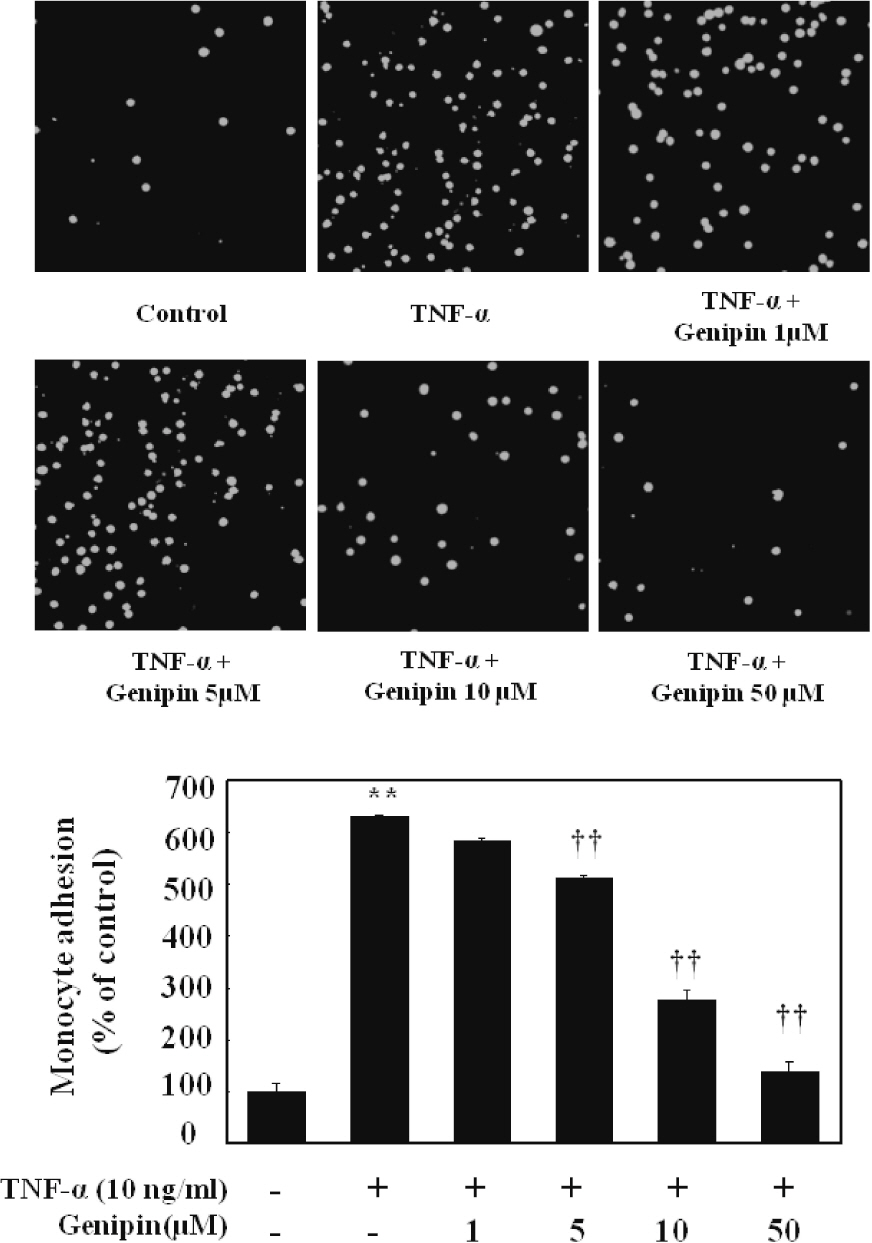
- Vascular inflammation process has been suggested to be an important risk factor in the development of atherosclerosis. Recently we reported that induction of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) selectively inhibits vascular cell adhesion molecule-1 (VCAM-1) but not intercellular cell adhesion molecule-1 (ICAM-1) in tumor necrosis factor (TNF)-alpha-activated human umbilical vein endothelial cells (HUVEC). In this study, we investigated whether genipin inhibits expression of cellular adhesion molecules, which is relevant to inflammation. Pretreatment with genipin reduced reactive oxygen species (ROS) production and expression of VCAM-1, but not ICAM-1 in TNF-alpha-activated HUVEC. Genipin dose- and time-dependently increased PPAR-gamma expression and inhibited TNF-alpha-induced phosphorylation of Akt and PKC with different degrees. Finally, genipin prevented TNF-alpha-induced adhesion of U937 monocytic cells to HUVEC. Taken together, these results indicate that upregualtion of PPAR-gamma by genipin selectively inhibits TNF-alpha-induced expression of VCAM-1, in which regulation of Akt and/or PKC play a key role. We concluded that genipin can be used for the treatment of cardiovascular disorders such as atherosclerosis.
Keyword
MeSH Terms
-
Atherosclerosis
Cell Adhesion
Endothelial Cells
Human Umbilical Vein Endothelial Cells
Humans
Inflammation
Intercellular Adhesion Molecule-1
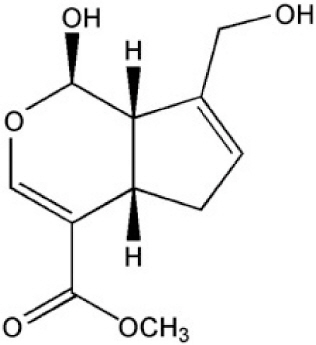
Iridoids
Peroxisomes
Phosphorylation
Reactive Oxygen Species
Risk Factors
Tumor Necrosis Factor-alpha
Up-Regulation
Vascular Cell Adhesion Molecule-1
Intercellular Adhesion Molecule-1
Iridoids
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
References
1. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999; 340:115–126.2. Fan J, Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003; 10:63–71.
Article3. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994; 76:301–314.
Article4. Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996; 271:13094–13102.5. Moon L, Ha YM, Jang HJ, Kim HS, Jun MS, Kim YM, Lee YS, Lee DH, Son KH, Kim HJ, Seo HG, Lee JH, Kim YS, Chang KC. Isoimperatorin, cimiside E and 23-O-acetylshengmanol-3-xyloside from Cimicifugae rhizome inhibit TNF-α-induced VCAM-1 expression in human endothelial cells: involvement of PPAR-γ upregulation and PI3K, ERK1/2, and PKC signal pathways. J Ethnopharmacol. 2011; 133:336–344.
Article6. Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Peroxisome proliferator-activated receptor activators target human endothelial cells to inhibit leukocyte-endothelial cell interaction. Arterioscler Thromb Vasc Biol. 1999; 19:2094–2104.
Article7. Nizamutdinova IT, Jeong JJ, Xu GH, Lee SH, Kang SS, Kim YS, Chang KC, Kim HJ. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells. Int Immunopharmacol. 2008; 8:670–678.8. Tsoyi K, Kim WS, Kim YM, Kim HJ, Seo HG, Lee JH, Yun-Choi HS, Chang KC. Upregulation of PTEN by CKD712, a synthetic tetrahydroisoquinoline alkaloid, selectively inhibits lipopoly-saccharide-induced VCAM-1 but not ICAM-1 expression in human endothelial cells. Atherosclerosis. 2009; 207:412–419.
Article9. Kim SJ, Kim JK, Lee DU, Kwak JH, Lee SM. Genipin protects lipopolysaccharide-induced apoptotic liver damage in D-galactosamine-sensitized mice. Eur J Pharmacol. 2010; 635:188–193.
Article10. Lee SJ, Oh PS, Lim KT. Hepatoprotective and hypolipidaemic effects of glycoprotein isolated from Gardenia jasminoides ellis in mice. Clin Exp Pharmacol Physiol. 2006; 33:925–933.
Article11. Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM, Kim SJ, Kim BC, Jin C, Lim CJ, Park EH. Antiinflammatory effects of genipin, an active principle of gardenia. Eur J Pharmacol. 2004; 495:201–208.
Article12. Desideri G, Ferri C. Endothelial activation. Sliding door to atherosclerosis. Curr Pharm Des. 2005; 11:2163–2175.
Article13. Albelda SM, Smith CW, Ward PA. Adhesion molecules and inflammatory injury. FASEB J. 1994; 8:504–512.
Article14. Sakai S, Kawamata H, Kogure T, Mantani N, Terasawa K, Umatake M, Ochiai H. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264.7 cells. Mediators Inflamm. 1999; 8:173–175.
Article15. Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator- activated receptor-gamma activators. Circulation. 2000; 101:235–238.16. Blaschke F, Caglayan E, Hsueh WA. Peroxisome proliferator-activated receptor gamma agonists: their role as vasoprotective agents in diabetes. Endocrinol Metab Clin North Am. 2006; 35:561–574.
Article17. Umetani M, Mataki C, Minegishi N, Yamamoto M, Hamakubo T, Kodama T. Function of GATA transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2001; 21:917–922.18. Nizamutdinova IT, Kim YM, Chung JI, Shin SC, Jeong YK, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats preferentially inhibit TNF-alpha-mediated induction of VCAM-1 over ICAM-1 through the regulation of GATAs and IRF-1. J Agric Food Chem. 2009; 57:7324–7330.19. Chen YH, Lin SJ, Chen YL, Liu PL, Chen JW. Anti-inflammatory effects of different drugs/agents with antioxidant property on endothelial expression of adhesion molecules. Cardiovasc Hematol Disord Drug Targets. 2006; 6:279–304.
Article20. Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001; 107:1255–1262.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allicin Reduces Adhesion Molecules and NO Production Induced by gamma irradiation in Human Endothelial Cells
- Cytokine Induced Differential Expression of Adhesion Molecules and HLA-DR in Cultured Human Glomerular Endothelial Cells
- Effect of Ultraviolet Light on the Expression of Adhesion Molecules and T Lymphocyte Adhesion to Human Dermal Microvascular Endothelial Cells
- Effects of mixed leukocyte reaction, hydrocortisone and cyclosporine on expression of leukocyte adhesion molecules by endothelial and mesangial cells
- Telmisartan Inhibits TNFα-Induced Leukocyte Adhesion by Blocking ICAM-1 Expression in Astroglial Cells but Not in Endothelial Cells