Anat Cell Biol.
2011 Sep;44(3):204-209. 10.5115/acb.2011.44.3.204.
Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma
- Affiliations
-
- 1Department of Anatomy, Pusan National University School of Medicine, Yangsan, Korea. hedgehog@pusan.ac.kr
- 2Medical Research Center for Ischemic Tissue Regeneration, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 1447432
- DOI: http://doi.org/10.5115/acb.2011.44.3.204
Abstract
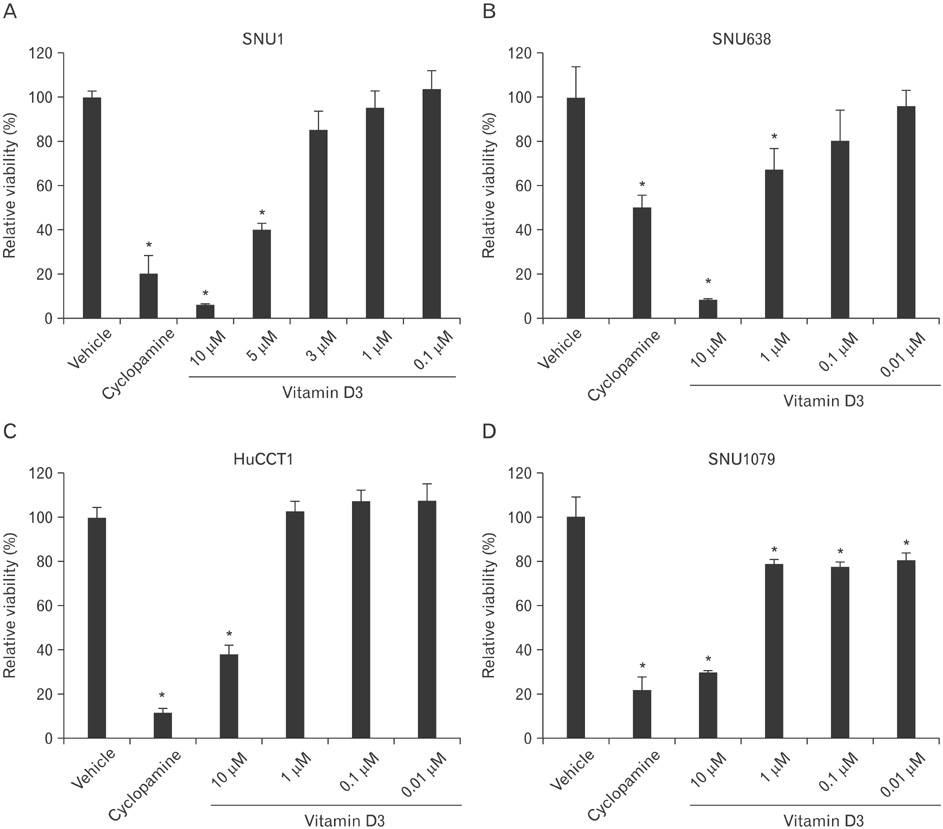
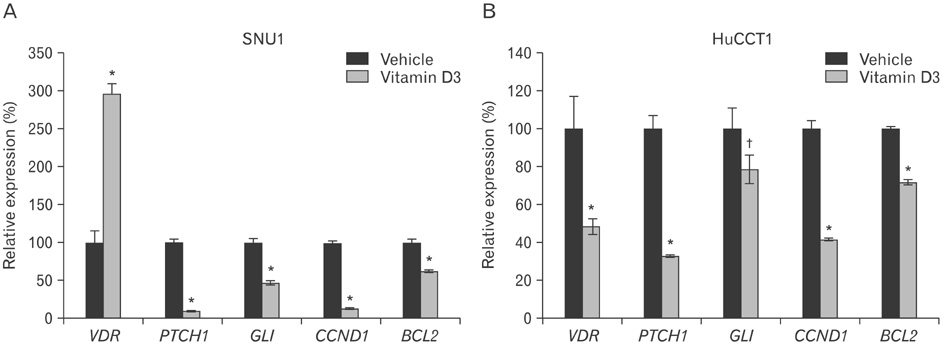
- A low serum level of vitamin D has been associated with an increased incidence of gastrointestinal tract cancers. However, the effects of vitamin D3 have not been investigated in gastric cancer and cholangiocarcinoma. In the present study, we found that vitamin D3 treatment significantly suppressed the viability of gastric cancer and cholangiocarcinoma cells. Moreover, vitamin D3 had a synergistic effect with other anti-cancer drugs, such as paclitaxel, adriamycin, and vinblastine, for suppressing cell viability. To determine the underlying mechanism involved in the regulation of viability by vitamin D3, we examined the effects of vitamin D3 on expression of hedgehog signaling target genes, which has been associated with gastric cancer and cholangiocarcinoma. Vitamin D3 treatment decreased the level of mRNA expression of patched1, Gli1, cyclin D1, and Bcl2, suggesting the possibility that vitamin D3 may act through regulation of hedgehog signaling. From the above results, we conclude that vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma.
MeSH Terms
Figure
Cited by 1 articles
-
Effect of meloxicam (cyclooygenase-2 inhibitor) versus vitamin D3 (cholecalciferol) as ameliorating agents of progressive doxorubicin-induced nephrotoxicity in rats
Dalia Mahmoud Abdelmonem Elsherbini, Hasnaa Ali Ebrahim
Anat Cell Biol. 2020;53(2):169-182. doi: 10.5115/acb.19.231.
Reference
-
1. Holick MF. Vitamin D and bone health. J Nutr. 1996. 126:4 Suppl. 1159S–1164S.2. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007. 7:684–700.3. Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001. 98:7498–7503.4. Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997. 16:391–396.5. Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997. 94:9831–9835.6. Garland CF, Comstock GW, Garland FC, Helsing KJ, Shaw EK, Gorham ED. Serum 25-hydroxyvitamin D and colon cancer: eight-year prospective study. Lancet. 1989. 2:1176–1178.7. Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005. 14:1991–1997.8. Ahonen MH, Tenkanen L, Teppo L, Hakama M, Tuohimaa P. Prostate cancer risk and prediagnostic serum 25-hydroxyvitamin D levels (Finland). Cancer Causes Control. 2000. 11:847–852.9. Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006. 98:451–459.10. Cross HS, Bises G, Lechner D, Manhardt T, Kállay E. The Vitamin D endocrine system of the guts: its possible role in colorectal cancer prevention. J Steroid Biochem Mol Biol. 2005. 97:121–128.11. Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol. 2006. 57:234–240.12. Mimori K, Tanaka Y, Yoshinaga K, Masuda T, Yamashita K, Okamoto M, Inoue H, Mori M. Clinical significance of the overexpression of the candidate oncogene CYP24 in esophageal cancer. Ann Oncol. 2004. 15:236–241.13. Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, Kuo WL, Gray JW, Pinkel D. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet. 2000. 25:144–146.14. Weiss MM, Snijders AM, Kuipers EJ, Ylstra B, Pinkel D, Meuwissen SG, van Diest PJ, Albertson DG, Meijer GA. Determination of amplicon boundaries at 20q13.2 in tissue samples of human gastric adenocarcinomas by high-resolution microarray comparative genomic hybridization. J Pathol. 2003. 200:320–326.15. Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005. 97:153–164.16. Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003. 3:592–600.17. Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006. 12:2979–2990.18. Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004. 66:167–179.19. Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004. 40:472–477.20. Tahara E. Genetic pathways of two types of gastric cancer. IARC Sci Publ. 2004. (157):327–349.21. Wise C, Pilanthananond M, Perry BF, Alpini G, McNeal M, Glaser SS. Mechanisms of biliary carcinogenesis and growth. World J Gastroenterol. 2008. 14:2986–2989.22. Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2010. 299:G1–G9.23. Rashid A. Cellular and molecular biology of biliary tract cancers. Surg Oncol Clin N Am. 2002. 11:995–1009.24. Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003. 425:846–851.25. Brüggemann LW, Queiroz KC, Zamani K, van Straaten A, Spek CA, Bijlsma MF. Assessing the efficacy of the hedgehog pathway inhibitor vitamin D3 in a murine xenograft model for pancreatic cancer. Cancer Biol Ther. 2010. 10:79–88.26. Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006. 4:e232.27. Han ME, Lee YS, Baek SY, Kim BS, Kim JB, Oh SO. Hedgehog signaling regulates the survival of gastric cancer cells by regulating the expression of Bcl-2. Int J Mol Sci. 2009. 10:3033–3043.28. Hathcock JN, Shao A, Vieth R, Heaney R. Risk assessment for vitamin D. Am J Clin Nutr. 2007. 85:6–18.29. Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008. 15:801–812.30. Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993. 75:1417–1430.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of Vitamin D(3) and TGF-beta on the viability of human periodontal ligament cells
- Expression and roles of NUPR1 in cholangiocarcinoma cells
- CDH3/P-Cadherin regulates migration of HuCCT1 cholangiocarcinoma cells
- Exploring optimal supplementation for people with vitamin D deficiency
- CD44 and CD133 as Cancer Stem Cell Markers for Gastric Cancer