Anat Cell Biol.
2010 Jun;43(2):110-117. 10.5115/acb.2010.43.2.110.
CDH3/P-Cadherin regulates migration of HuCCT1 cholangiocarcinoma cells
- Affiliations
-
- 1Department of Anatomy, School of Medicine, Pusan National University, Yangsan, Korea. hedgehog@pusan.ac.kr
- 2Medical Research Center for Ischemic Tissue Regeneration, Pusan National University, Yangsan, Korea.
- KMID: 2168883
- DOI: http://doi.org/10.5115/acb.2010.43.2.110
Abstract
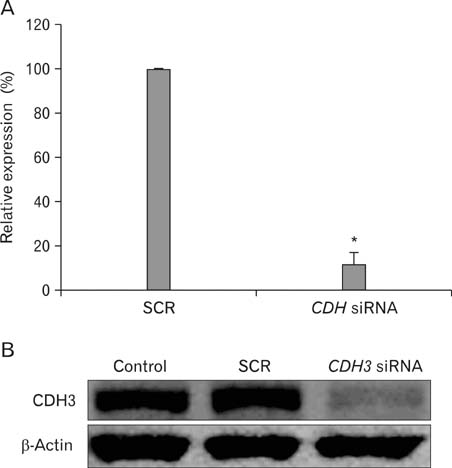
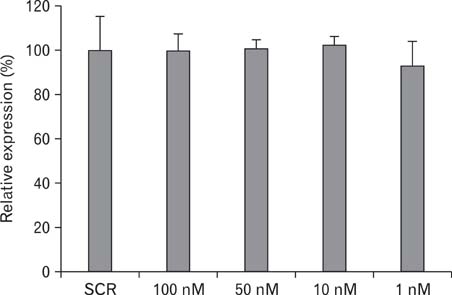
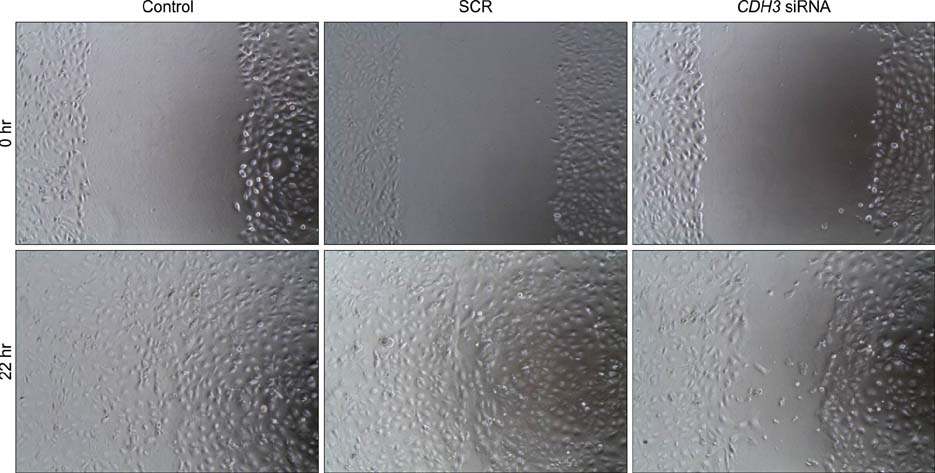
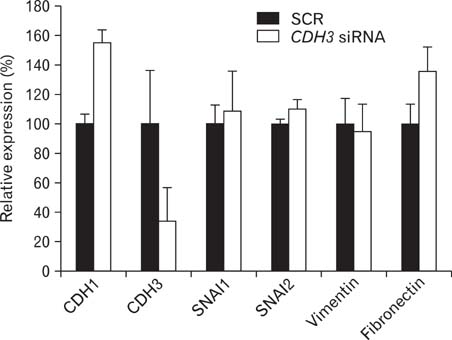
- Intrahepatic cholangiocarcinoma is the second most common subtype of primary hepatobilliary cancer. Despite advances in surgical and medical therapy, its survival rate remains poor. Compared to hepatocellular carcinoma (HCC), the most common liver malignancy, the underlying mechanisms of cholangiocarcinoma carcinogenesis are poorly characterized. P-cadherin (CDH3) is a cadherin super family member. Although CDH3 is frequently over-expressed in cholangiocarcinoma tissues, its roles have never been characterized. To determine the roles of CDH3 in cholangiocarcinoma, we investigated CDH3 function in HuCCT1 cells using specific siRNA. Transfection with CDH3 siRNA did not affect proliferation of HuCCT1 cells. However, cell migration and invasion were significantly reduced when CDH3 was down-regulated. In addition, expressions of several biomarkers for epithelial-mesenchymal transition (EMT) were not changed by CDH3 down-regulation. These results suggest that CDH3 regulates cell migration independent of EMT in cholangiocarcinoma cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Anastasiadis PZ, Reynolds AB. Regulation of Rho GTPases by p120-catenin. Curr Opin Cell Biol. 2001. 13:604–610.2. Cheung LW, Leung PC, Wong AS. Cadherin switching and activation of p120 catenin signaling are mediators of gonadotropin-releasing hormone to promote tumor cell migration and invasion in ovarian cancer. Oncogene. 2010. 29:2427–2440.3. Dasgupta S, Tripathi PK, Qin H, Bhattacharya-Chatterjee M, Valentino J, Chatterjee SK. Identification of molecular targets for immunotherapy of patients with head and neck squamous cell carcinoma. Oral Oncol. 2006. 42:306–316.4. Gamallo C, Moreno-Bueno G, Sarrió D, Calero F, Hardisson D, Palacios J. The prognostic significance of P-cadherin in infiltrating ductal breast carcinoma. Mod Pathol. 2001. 14:650–654.5. Gorski JJ, James CR, Quinn JE, et al. BRCA1 transcriptionally regulates genes associated with the basal-like phenotype in breast cancer. Breast Cancer Res Treat. 2009. [Epub ahead of print].6. Haÿ E, Nouraud A, Marie PJ. N-cadherin negatively regulates osteoblast proliferation and survival by antagonizing Wnt, ERK and PI3K/Akt signalling. PLoS One. 2009. 4:e8284.7. Hibi K, Goto T, Mizukami H, et al. Demethylation of the CDH3 gene is frequently detected in advanced colorectal cancer. Anticancer Res. 2009a. 29:2215–2217.8. Hibi K, Kitamura YH, Mizukami H, et al. Frequent CDH3 demethylation in advanced gastric carcinoma. Anticancer Res. 2009b. 29:3945–3947.9. Imai K, Hirata S, Irie A, et al. Identification of a novel tumor-associated antigen, cadherin 3/P-cadherin, as a possible target for immunotherapy of pancreatic, gastric, and colorectal cancers. Clin Cancer Res. 2008. 14:6487–6495.10. Jeon TY, Han ME, Lee YW, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010. 102:710–718.11. Kato I, Kuroishi T, Tominaga S. Descriptive epidemiology of subsites of cancers of the liver, biliary tract and pancreas in Japan. Jpn J Clin Oncol. 1990. 20:232–237.12. Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of ras-transformed breast epithelia. J Cell Biol. 1995. 130:461–471.13. Milicic A, Harrison LA, Goodlad RA, et al. Ectopic expression of P-cadherin correlates with promoter hypomethylation early in colorectal carcinogenesis and enhanced intestinal crypt fission in vivo. Cancer Res. 2008. 68:7760–7768.14. Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986. 103:2649–2658.15. Obama K, Ura K, Li M, et al. Genome-wide analysis of gene expression in human intrahepatic cholangiocarcinoma. Hepatology. 2005. 41:1339–1348.16. Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004. 66:167–179.17. Paredes J, Albergaria A, Oliveira JT, Jerónimo C, Milanezi F, Schmitt FC. P-cadherin overexpression is an indicator of clinical outcome in invasive breast carcinomas and is associated with CDH3 promoter hypomethylation. Clin Cancer Res. 2005. 11:5869–5877.18. Paredes J, Correia AL, Ribeiro AS, Milanezi F, Cameselle-Teijeiro J, Schmitt FC. Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. J Clin Pathol. 2008. 61:856–862.19. Pece S, Gutkind JS. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem. 2000. 275:41227–41233.20. Peralta Soler A, Knudsen KA, Salazar H, Han AC, Keshgegian AA. P-cadherin expression in breast carcinoma indicates poor survival. Cancer. 1999. 86:1263–1272.21. Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J. 2004. 23:1739–1748.22. Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004. 40:472–477.23. Simpson KJ, Selfors LM, Bui J, et al. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008. 10:1027–1038.24. Staddon JM, Smales C, Schulze C, Esch FS, Rubin LL. p120, a p120-related protein (p100), and the cadherin/catenin complex. J Cell Biol. 1995. 130:369–381.25. Stefansson IM, Salvesen HB, Akslen LA. Prognostic impact of alterations in P-cadherin expression and related cell adhesion markers in endometrial cancer. J Clin Oncol. 2004. 22:1242–1252.26. Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002. 2:301–314.27. Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995. 7:619–627.28. Taniuchi K, Nakagawa H, Hosokawa M, et al. Overexpressed P-cadherin/CDH3 promotes motility of pancreatic cancer cells by interacting with p120ctn and activating rho-family GTPases. Cancer Res. 2005. 65:3092–3099.29. Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979-94. Lancet. 1997. 350:1142–1143.30. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009. 139:871–890.31. van Roy FM, McCrea PD. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer. 2005. 5:956–964.32. Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009. 28:15–33.33. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009. 119:1429–1437.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression and roles of NUPR1 in cholangiocarcinoma cells
- Toll-Like Receptor-Mediated Free Radical Generation in Clonorchis sinensis Excretory-Secretory Product-Treated Cholangiocarcinoma Cells
- Sphingosylphosphorylcholine Induces Thrombospondin-1 Secretion in MCF10A Cells via ERK2
- Synovial Cell Migration is Associated with B Cell Activating Factor Expression Increased by TNFα or Decreased by KR33426
- Regulation of V-cadherin Expression on Human Dermal Microvascular Endothelial Cells