Anat Cell Biol.
2012 Mar;45(1):17-25. 10.5115/acb.2012.45.1.17.
Expression and roles of NUPR1 in cholangiocarcinoma cells
- Affiliations
-
- 1Department of Anatomy, Pusan National University School of Medicine, Yangsan, Korea. hedgehog@pusan.ac.kr
- 2Medical Research Center for Ischemic Tissue Regeneration, Pusan National University, Yangsan, Korea.
- KMID: 1447449
- DOI: http://doi.org/10.5115/acb.2012.45.1.17
Abstract
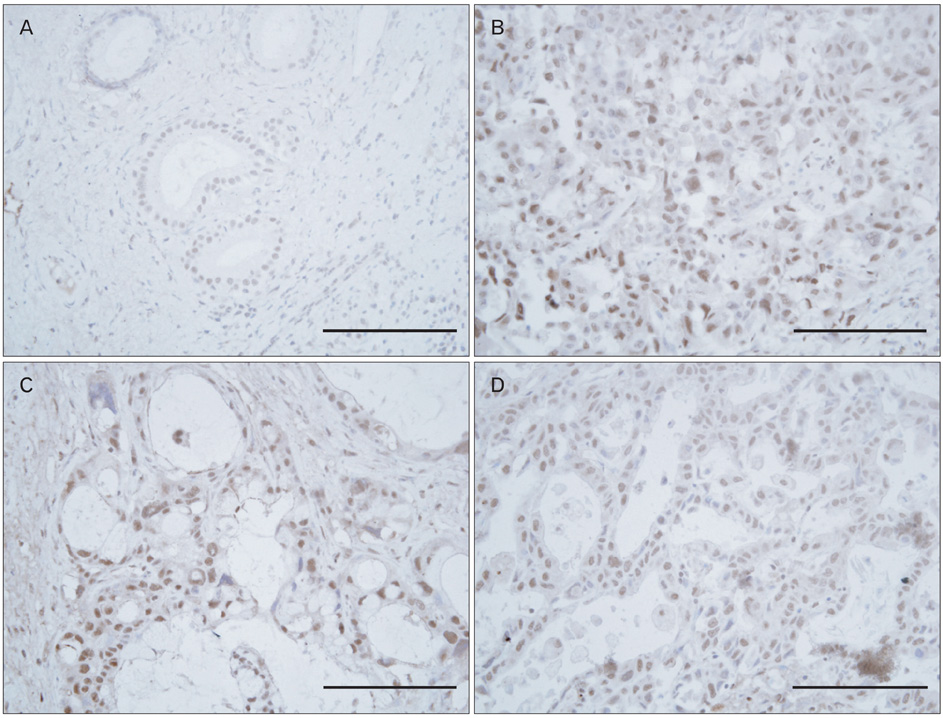
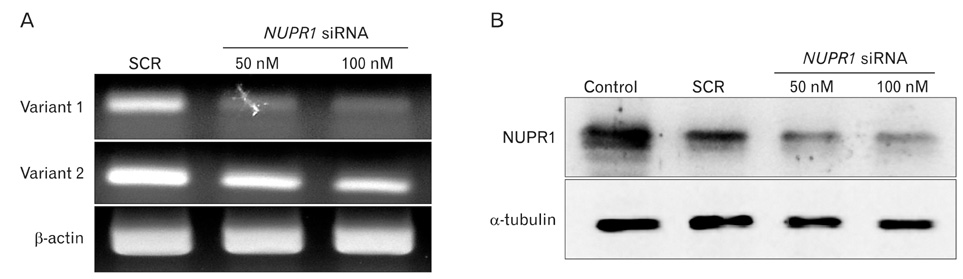
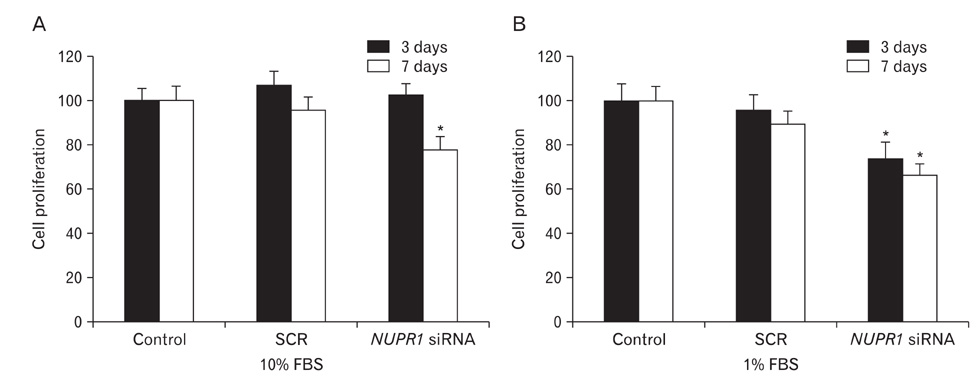
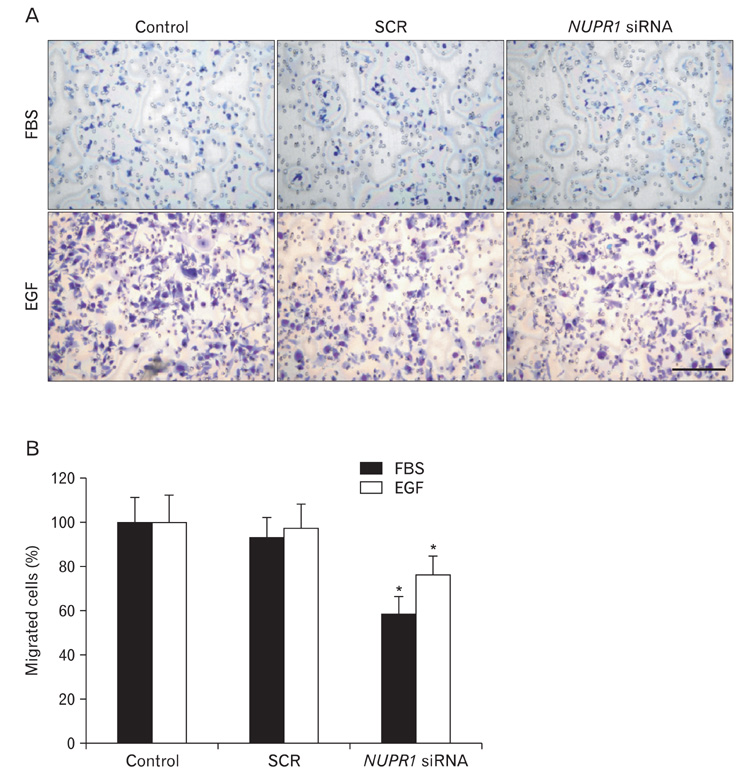
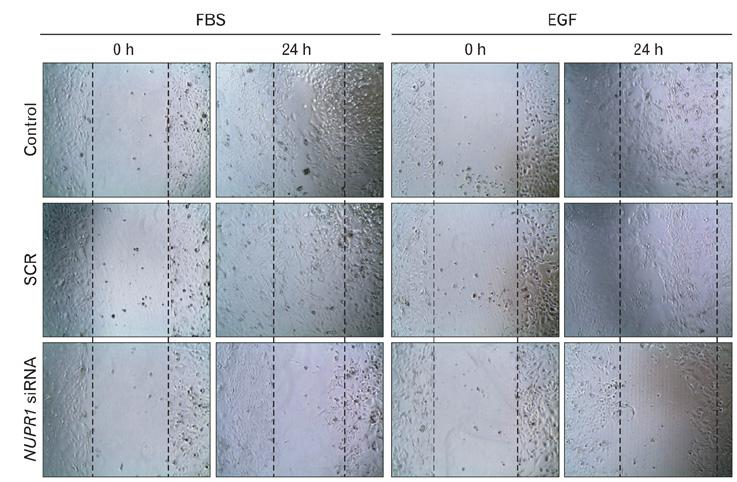
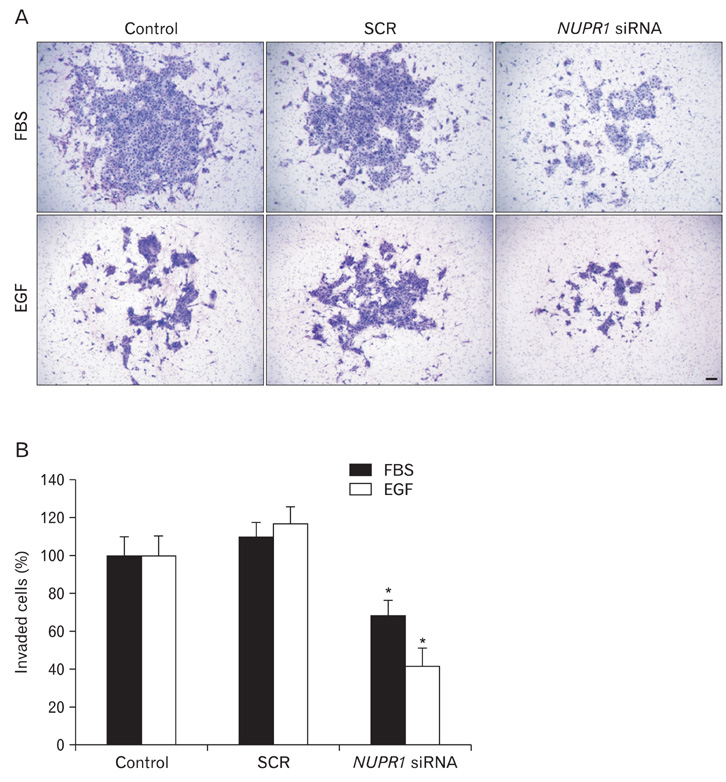
- Nuclear protein-1 (NUPR1) is a small nuclear protein that is responsive to various stress stimuli. Although NUPR1 has been associated with cancer development, its expression and roles in cholangiocarcinoma have not yet been described. In the present study, we found that NUPR1 was over-expressed in human cholangiocarcinoma tissues, using immunohistochemistry. The role of NUPR1 in cholangiocarcinoma was examined by its specific siRNA. NUPR1 siRNA decreased proliferation, migration and invasion of human cholangiocarcinoma cell lines (HuCCT1 and SNU1196 cells). From these results, we conclude that NUPR1 is over-expressed in cholangiocarcinoma and regulates the proliferation and motility of cancer cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Chowdhury UR, Samant RS, Fodstad O, Shevde LA. Emerging role of nuclear protein 1 (NUPR1) in cancer biology. Cancer Metastasis Rev. 2009. 28:225–232.2. Goruppi S, Iovanna JL. Stress-inducible protein p8 is involved in several physiological and pathological processes. J Biol Chem. 2010. 285:1577–1581.3. Cano CE, Hamidi T, Sandi MJ, Iovanna JL. Nupr1: the Swiss-knife of cancer. J Cell Physiol. 2011. 226:1439–1443.4. Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. J Biol Chem. 1997. 272:32360–32369.5. Jiang YF, Vaccaro MI, Fiedler F, Calvo EL, Iovanna JL. Lipopolysaccharides induce p8 mRNA expression in vivo and in vitro. Biochem Biophys Res Commun. 1999. 260:686–690.6. Taïeb D, Malicet C, Garcia S, Rocchi P, Arnaud C, Dagorn JC, Iovanna JL, Vasseur S. Inactivation of stress protein p8 increases murine carbon tetrachloride hepatotoxicity via preserved CYP2E1 activity. Hepatology. 2005. 42:176–182.7. Plant SR, Wang Y, Vasseur S, Thrash JC, McMahon EJ, Bergstralh DT, Arnett HA, Miller SD, Carson MJ, Iovanna JL, Ting JP. Upregulation of the stress-associated gene p8 in mouse models of demyelination and in multiple sclerosis tissues. Glia. 2006. 53:529–537.8. Zinke I, Schütz CS, Katzenberger JD, Bauer M, Pankratz MJ. Nutrient control of gene expression in Drosophila: microarray analysis of starvation and sugar-dependent response. EMBO J. 2002. 21:6162–6173.9. Valacco MP, Varone C, Malicet C, Cánepa E, Iovanna JL, Moreno S. Cell growth-dependent subcellular localization of p8. J Cell Biochem. 2006. 97:1066–1079.10. García-Montero AC, Vasseur S, Giono LE, Canepa E, Moreno S, Dagorn JC, Iovanna JL. Transforming growth factor beta-1 enhances Smad transcriptional activity through activation of p8 gene expression. Biochem J. 2001. 357(Pt 1):249–253.11. Goruppi S, Patten RD, Force T, Kyriakis JM. Helix-loop-helix protein p8, a transcriptional regulator required for cardio myocyte hypertrophy and cardiac fibroblast matrix metalloprotease induction. Mol Cell Biol. 2007. 27:993–1006.12. Path G, Opel A, Knoll A, Seufert J. Nuclear protein p8 is associated with glucose-induced pancreatic beta-cell growth. Diabetes. 2004. 53:Suppl 1. S82–S85.13. Carracedo A, Lorente M, Egia A, Blázquez C, García S, Giroux V, Malicet C, Villuendas R, Gironella M, González-Feria L, Piris MA, Iovanna JL, Guzmán M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006. 9:301–312.14. Goruppi S, Bonventre JV, Kyriakis JM. Signaling pathways and late-onset gene induction associated with renal mesangial cell hypertrophy. EMBO J. 2002. 21:5427–5436.15. Million Passe CM, White CR, King MW, Quirk PL, Iovanna JL, Quirk CC. Loss of the protein NUPR1 (p8) leads to delayed LHB expression, delayed ovarian maturation, and testicular development of a sertoli-cell-only syndrome-like phenotype in mice. Biol Reprod. 2008. 79:598–607.16. Vasseur S, Mallo GV, Garcia-Montero A, Ortiz EM, Fiedler F, Cánepa E, Moreno S, Iovanna JL. Structural and functional characterization of the mouse p8 gene: promotion of transcription by the CAAT-enhancer binding protein alpha (C/EBPalpha) and C/EBPbeta trans-acting factors involves a C/EBP cisacting element and other regions of the promoter. Biochem J. 1999. 343(Pt 2):377–383.17. Goruppi S, Kyriakis JM. The pro-hypertrophic basic helix-loop-helix protein p8 is degraded by the ubiquitin/proteasome system in a protein kinase B/Akt- and glycogen synthase kinase-3-dependent manner, whereas endothelin induction of p8 mRNA and renal mesangial cell hypertrophy require NFAT4. J Biol Chem. 2004. 279:20950–20958.18. Malicet C, Giroux V, Vasseur S, Dagorn JC, Neira JL, Iovanna JL. Regulation of apoptosis by the p8/prothymosin alpha complex. Proc Natl Acad Sci U S A. 2006. 103:2671–2676.19. Hoffmeister A, Ropolo A, Vasseur S, Mallo GV, Bodeker H, Ritz-Laser B, Dressler GR, Vaccaro MI, Dagorn JC, Moreno S, Iovanna JL. The HMG-I/Y-related protein p8 binds to p300 and Pax2 trans-activation domain-interacting protein to regulate the trans-activation activity of the Pax2A and Pax2B transcription factors on the glucagon gene promoter. J Biol Chem. 2002. 277:22314–22319.20. Sandi MJ, Hamidi T, Malicet C, Cano C, Loncle C, Pierres A, Dagorn JC, Iovanna JL. p8 expression controls pancreatic cancer cell migration, invasion, adhesion, and tumorigenesis. J Cell Physiol. 2011. 226:3442–3451.21. Vasseur S, Hoffmeister A, Garcia S, Bagnis C, Dagorn JC, Iovanna JL. p8 is critical for tumour development induced by rasV12 mutated protein and E1A oncogene. EMBO Rep. 2002. 3:165–170.22. Ree AH, Pacheco MM, Tvermyr M, Fodstad O, Brentani MM. Expression of a novel factor, com1, in early tumor progression of breast cancer. Clin Cancer Res. 2000. 6:1778–1783.23. Ree AH, Tvermyr M, Engebraaten O, Rooman M, Røsok O, Hovig E, Meza-Zepeda LA, Bruland OS, Fodstad O. Expression of a novel factor in human breast cancer cells with metastatic potential. Cancer Res. 1999. 59:4675–4680.24. Ito Y, Yoshida H, Motoo Y, Iovanna JL, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A. Expression of p8 protein in medullary thyroid carcinoma. Anticancer Res. 2005. 25:3419–3423.25. Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004. 66:167–179.26. Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010. 101:579–585.27. Lim MK, Ju YH, Franceschi S, Oh JK, Kong HJ, Hwang SS, Park SK, Cho SI, Sohn WM, Kim DI, Yoo KY, Hong ST, Shin HR. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006. 75:93–96.28. Kurathong S, Lerdverasirikul P, Wongpaitoon V, Pramoolsinsap C, Kanjanapitak A, Varavithya W, Phuapradit P, Bunyaratvej S, Upatham ES, Brockelman WY. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology. 1985. 89:151–156.29. Baek S, Lee YW, Yoon S, Baek SY, Kim BS, Oh SO. CDH3/P-Cadherin regulates migration of HuCCT1 cholangiocarcinoma cells. Anat Cell Biol. 2010. 43:110–117.30. Ree AH, Bratland A, Kroes RA, Aasheim HC, Flørenes VA, Moskal JR, Fodstad O, Bruland OS, Maelandsmo GM. Clinical and cell line specific expression profiles of a human gene identified in experimental central nervous system metastases. Anticancer Res. 2002. 22:1949–1957.31. Brannon KM, Million Passe CM, White CR, Bade NA, King MW, Quirk CC. Expression of the high mobility group A family member p8 is essential to maintaining tumorigenic potential by promoting cell cycle dysregulation in LbetaT2 cells. Cancer Lett. 2007. 254:146–155.32. Jiang WG, Davies G, Martin TA, Kynaston H, Mason MD, Fodstad O. Com-1/p8 acts as a putative tumour suppressor in prostate cancer. Int J Mol Med. 2006. 18:981–986.33. Jiang WG, Davies G, Kynaston H, Mason MD, Fodstad O. Does the PGC-1/PPARgamma pathway play a role in Com-1/p8 mediated cell growth inhibition in prostate cancer? Int J Mol Med. 2006. 18:1169–1175.34. Sambasivan R, Cheedipudi S, Pasupuleti N, Saleh A, Pavlath GK, Dhawan J. The small chromatin-binding protein p8 coordinates the association of anti-proliferative and pro-myogenic proteins at the myogenin promoter. J Cell Sci. 2009. 122(Pt 19):3481–3491.35. Sambasivan R, Pavlath GK, Dhawan J. A gene-trap strategy identifies quiescence-induced genes in synchronized myoblasts. J Biosci. 2008. 33:27–44.36. Malicet C, Hoffmeister A, Moreno S, Closa D, Dagorn JC, Vasseur S, Iovanna JL. Interaction of the stress protein p8 with Jab1 is required for Jab1-dependent p27 nuclear-to-cytoplasm translocation. Biochem Biophys Res Commun. 2006. 339:284–289.37. Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003. 3:362–374.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CDH3/P-Cadherin regulates migration of HuCCT1 cholangiocarcinoma cells
- Molecular pathogenesis and the role of tumor markers in cholangiocarcinoma
- Clinical Significance of the Survivin Expression in Intrahepatic Cholangiocarcinoma with Hepatolithiasis
- Expression of Epidermal Growth Factor Receptor, ErbB2 and Matrix Metalloproteinase-9 in Hepatolithiasis and Cholangiocarcinoma
- Impact of Nrf2 overexpression on cholangiocarcinoma treatment and clinical prognosis