Histologic effects of intentional-socket-assisted orthodontic movement in rabbits
- Affiliations
-
- 1Graduate School of Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Oral and Maxillofacial Surgery, Uijeongbu St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 3Postgraduate Orthodontic Program, Arizona School of Dentistry & Oral Health, A.T. Still University, Mesa, AZ, USA.
- 4Graduate School of Dentistry, Kyung Hee University, Seoul, Korea.
- 5Department of Orthodontics, The Catholic University of Korea School of Medicine, Seoul, Korea.
- 6Dental Focus, Sydney, NSW, Australia.
- 7Department of Orthodontics, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. kook190036@yahoo.com
- KMID: 1435415
- DOI: http://doi.org/10.4041/kjod.2012.42.4.207
Abstract
OBJECTIVE
This study aimed to evaluate the effect of an intentionally created socket on bone remodeling with orthodontic tooth movement in rabbits.
METHODS
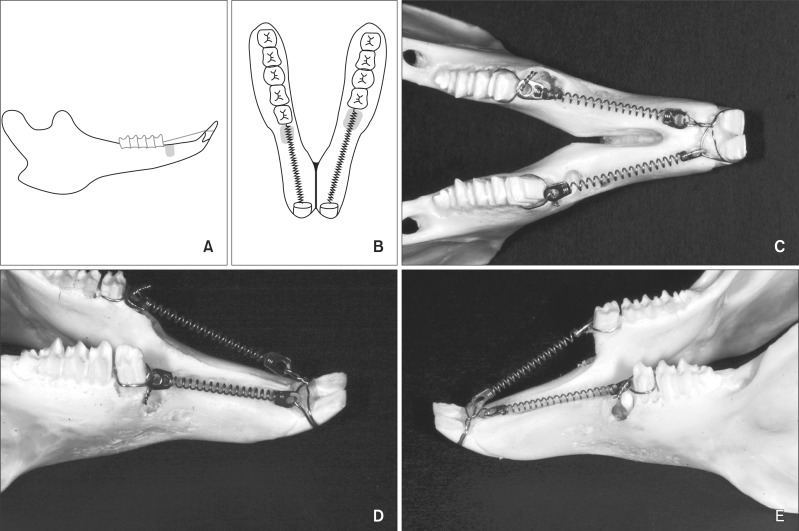
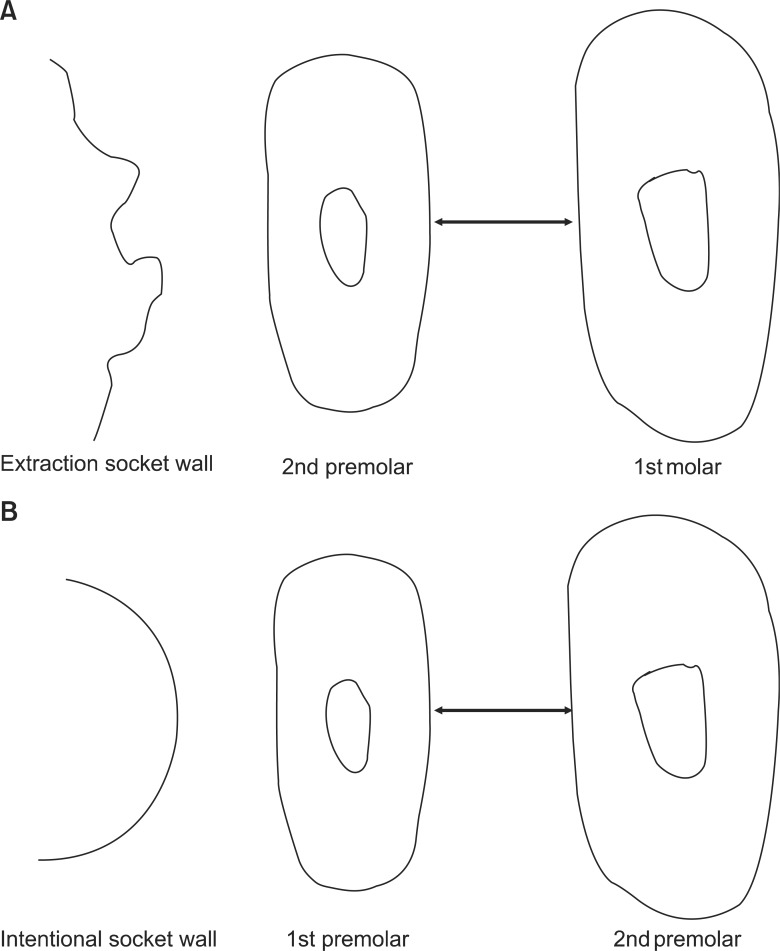
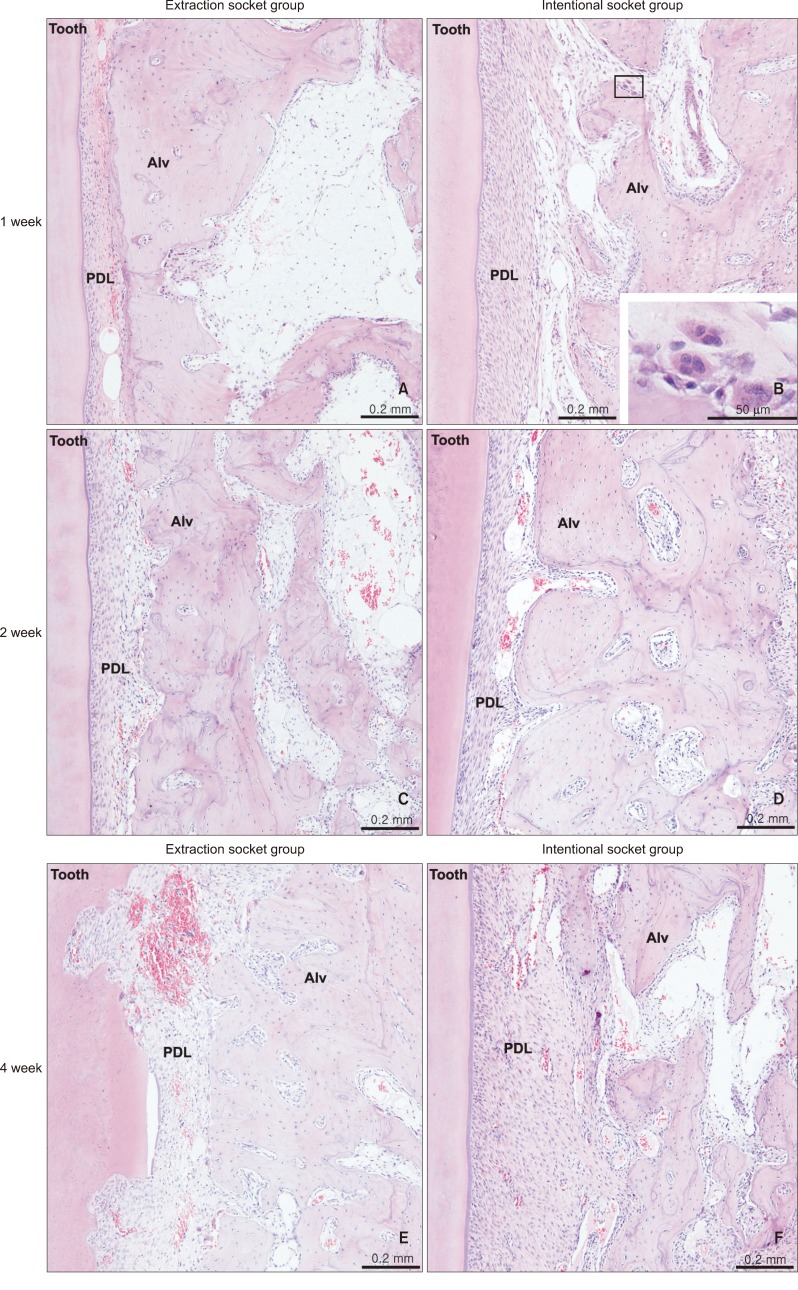
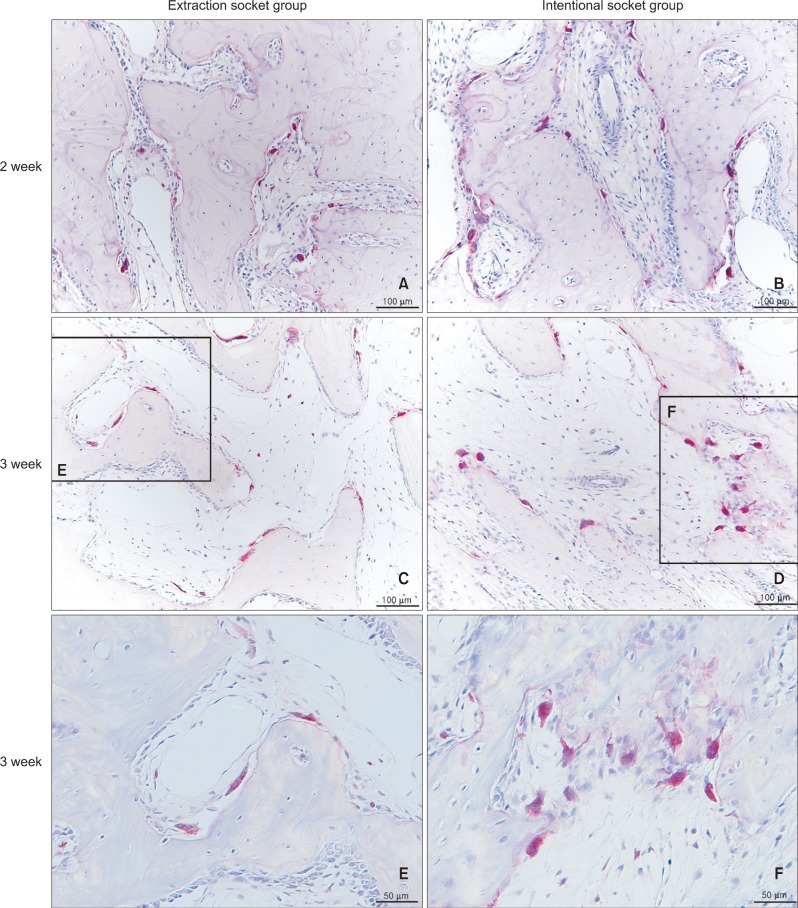
Eighteen male rabbits weighing 3.8 - 4.25 kg were used. An 8-mm deep and 2-mm wide socket was drilled in the bone 1 mm mesial to the right mandibular first premolar. The left first premolar was extracted to serve as an extraction socket. A traction force of 100 cN was applied to the right first premolar and left second premolar. Sections were obtained at the middle third of the moving tooth for both the drilled and extraction sockets and evaluated with hematoxylin and eosin staining and immunohistochemical analyses. The amount of tooth movement and tartrate-resistant acid phosphatase (TRAP)-positive cell count were compared between the 2 groups using the Mann-Whitney U test.
RESULTS
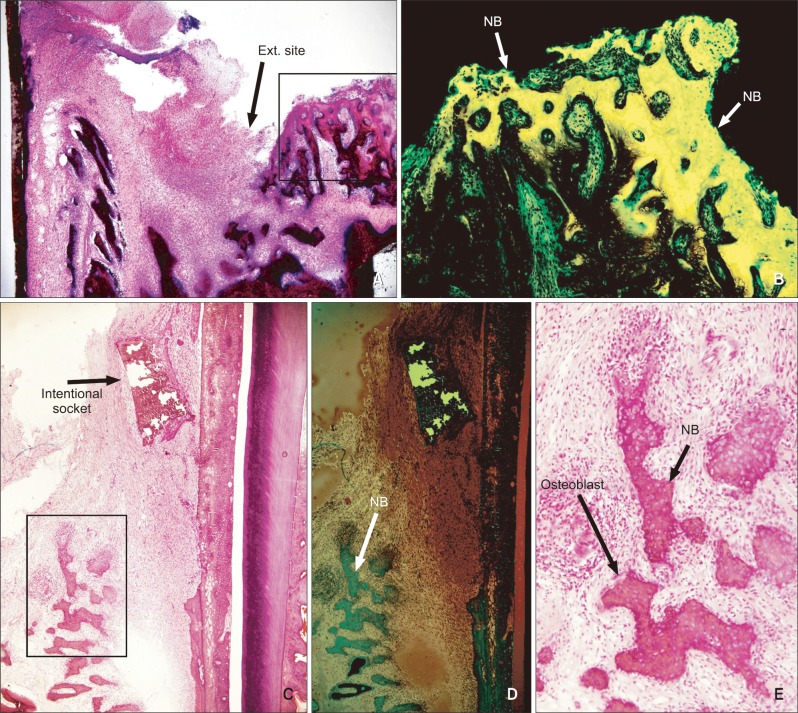
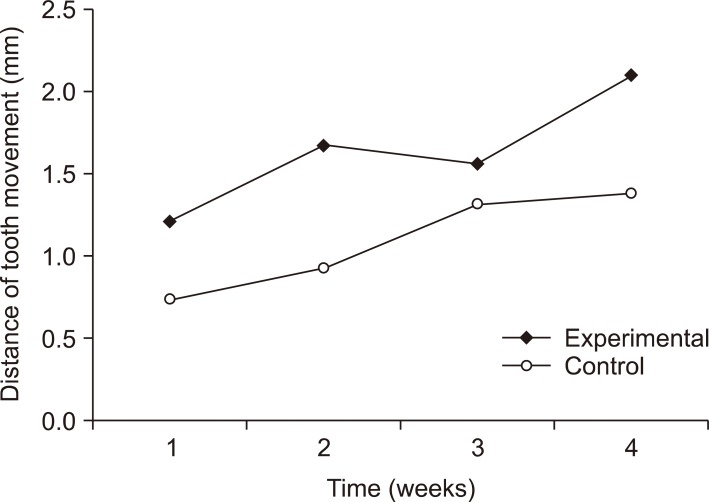
At week 2, the distance of tooth movement was significantly higher in the intentional socket group (p < 0.05) than in the extraction socket group. The number of TRAP-positive cells decreased in week 2 but increased in week 3 (p < 0.05). However, there were no significant differences between the groups. Furthermore, results of transforming growth factor (TGF)-beta staining revealed no significant differences.
CONCLUSIONS
The intentional socket group showed greater distance of tooth movement than did the extraction socket group at week 2. Osteoclast counts and results of immunohistochemical analyses suggested elevated bone remodeling in both the groups. Thus, osteotomy may be an effective modality for enhancing tooth movement in orthodontic treatment.
MeSH Terms
Figure
Cited by 3 articles
-
Comparison of tooth movement and biological response in corticotomy and micro-osteoperforation in rabbits
Junghan Kim, Yoon-Ah Kook, Mohamed Bayome, Jae Hyun Park, Won Lee, Hojae Choi, Noha H. Abbas
Korean J Orthod. 2019;49(4):205-213. doi: 10.4041/kjod.2019.49.4.205.Comparison of the effects of horizontal and vertical micro-osteoperforations on the biological response and tooth movement in rabbits
Seok-gon Kim, Yoon-Ah Kook, Hee Jin Lim, Patrick Park, Won Lee, Jae Hyun Park, Mohamed Bayome, Yoonji Kim
Korean J Orthod. 2021;51(5):304-312. doi: 10.4041/kjod.2021.51.5.304.Alveolar ridge expansion-assisted orthodontic space closure in the mandibular posterior region
Mete Ozer, Berat Serdar Akdeniz, Mahmut Sumer
Korean J Orthod. 2013;43(6):302-310. doi: 10.4041/kjod.2013.43.6.302.
Reference
-
1. Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006; 129:469. PMID: 16627171.
Article2. Chumbley AB, Tuncay OC. The effect of indomethacin (an aspirin-like drug) on the rate of orthodontic tooth movement. Am J Orthod. 1986; 89:312–314. PMID: 3083687.3. Chung KR, Oh MY, Ko SJ. Corticotomy-assisted orthodontics. J Clin Orthod. 2001; 35:331–339. PMID: 11475544.4. Iino S, Sakoda S, Ito G, Nishimori T, Ikeda T, Miyawaki S. Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am J Orthod Dentofacial Orthop. 2007; 131:448. PMID: 17418709.
Article5. Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959; 12:515–529. PMID: 13644913.6. Lee WC. Experimental study of the effect of prostaglandin administration on tooth movement--with particular emphasis on the relationship to the method of PGE1 administration. Am J Orthod Dentofacial Orthop. 1990; 98:231–241. PMID: 2403075.
Article7. Stark TM, Sinclair PM. Effect of pulsed electromagnetic fields on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1987; 91:91–104. PMID: 3468800.
Article8. Wilcko WM, Ferguson DJ, Bouquot JE, Wilcko MT. Rapid orthodontic decrowding with alveolar augmentation: case report. World J Orthod. 2003; 4:197–205.9. Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001; 21:9–19. PMID: 11829041.10. Converse JM, Horowitz SL. The surgical-orthodontic approach to the treatment of dentofacial deformities. Am J Orthod. 1969; 55:217–243. PMID: 4886159.
Article11. Işeri H, Kişnişci R, Bzizi N, Tüz H. Rapid canine retraction and orthodontic treatment with dentoalveolar distraction osteogenesis. Am J Orthod Dentofacial Orthop. 2005; 127:533–541. PMID: 15877033.12. Sukurica Y, Karaman A, Gürel HG, Dolanmaz D. Rapid canine distalization through segmental alveolar distraction osteogenesis. Angle Orthod. 2007; 77:226–236. PMID: 17319756.
Article13. Sebaoun JD, Kantarci A, Turner JW, Carvalho RS, Van Dyke TE, Ferguson DJ. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008; 79:1679–1688. PMID: 18771369.
Article14. Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011; 139(4 Suppl):S83–S101. PMID: 21435543.
Article15. Mostafa YA, Mohamed Salah Fayed M, Mehanni S, ElBokle NN, Heider AM. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofacial Orthop. 2009; 136:570–577. PMID: 19815161.
Article16. Ren A, Lv T, Kang N, Zhao B, Chen Y, Bai D. Rapid orthodontic tooth movement aided by alveolar surgery in beagles. Am J Orthod Dentofacial Orthop. 2007; 131:160. PMID: 17276852.
Article17. Wang L, Lee W, Lei DL, Liu YP, Yamashita DD, Yen SL. Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: histology and immunostaining. Am J Orthod Dentofacial Orthop. 2009; 136:770. PMID: 19962598.
Article18. Chung KR, Mitsugi M, Lee BS, Kanno T, Lee W, Kim SH. Speedy surgical orthodontic treatment with skeletal anchorage in adults--sagittal correction and open bite correction. J Oral Maxillofac Surg. 2009; 67:2130–2148. PMID: 19761907.
Article19. Seifi M, Shafeei HA, Daneshdoost S, Mir M. Effects of two types of low-level laser wave lengths (850 and 630 nm) on the orthodontic tooth movements in rabbits. Lasers Med Sci. 2007; 22:261–264. PMID: 17334676.
Article20. Kim T, Handa A, Iida J, Yoshida S. RANKL expression in rat periodontal ligament subjected to a continuous orthodontic force. Arch Oral Biol. 2007; 52:244–250. PMID: 17101113.
Article21. Verna C, Zaffe D, Siciliani G. Histomorphometric study of bone reactions during orthodontic tooth movement in rats. Bone. 1999; 24:371–379. PMID: 10221549.
Article22. Park WK, Kim SS, Park SB, Son WS, Kim YD, Jun ES, et al. The effect of cortical punching on the expression of OPG, RANK, and RANKL in the periodontal tissue during tooth movement in rats. Korean J Orthod. 2008; 38:159–174.
Article23. Kiliç N, Oktay H, Ersöz M. Effects of force magnitude on tooth movement: an experimental study in rabbits. Eur J Orthod. 2010; 32:154–158. PMID: 19740977.24. Kilic N, Oktay H, Ersoz M. Effects of force magnitude on relapse: An experimental study in rabbits. Am J Orthod Dentofacial Orthop. 2011; 140:44–50. PMID: 21724086.
Article26. Barone A, Aldini NN, Fini M, Giardino R, Calvo Guirado JL, Covani U. Xenograft versus extraction alone for ridge preservation after tooth removal: a clinical and histomorphometric study. J Periodontol. 2008; 79:1370–1377. PMID: 18672985.
Article27. Herr Y, Matsuura M, Lin WL, Genco RJ, Cho MI. The origin of fibroblasts and their role in the early stages of horizontal furcation defect healing in the beagle dog. J Periodontol. 1995; 66:716–730. PMID: 7473015.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgically assisted orthodontic treatment of ankylosed maxillary incisor
- Histopathologic investigation of the effects of prostaglandin E2 administered by different methods on tooth movement and bone metabolism
- Intentional replantation with preapplication of orthodontic force on mandibular second molar
- The influence of leukocyte-platelet-rich plasma on accelerated orthodontic tooth movement in rabbits
- Mode of tooth movement according to the timing of orthodontic force application after extraction