Korean J Orthod.
2012 Jun;42(3):118-128. 10.4041/kjod.2012.42.3.118.
Histopathologic investigation of the effects of prostaglandin E2 administered by different methods on tooth movement and bone metabolism
- Affiliations
-
- 1Department of Orthodontics, Dentistry Faculty, Kirikkale University, Kirikkale, Turkey. drcaglaroglu@kku.edu.tr
- 2Department of Orthodontics, Dentistry Faculty, Ataturk University, Erzurum, Turkey.
- KMID: 2272260
- DOI: http://doi.org/10.4041/kjod.2012.42.3.118
Abstract
OBJECTIVE
The aim of this study was to investigate and compare the in vivo effects of prostaglandin E2 (PGE2) administered by different methods on orthodontic tooth movement and bone metabolism macroscopically, histopatologically, and biochemically.
METHODS
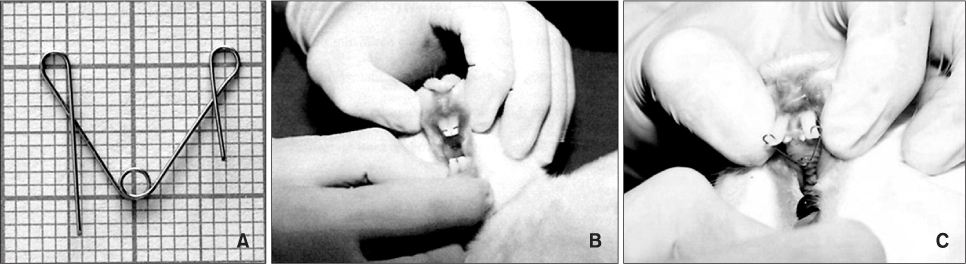
Forty-five young adult New Zealand rabbits were randomly divided into 3 experimental groups (n = 10/group), 1 positive control group (n = 10), and 1 negative control group (n = 5). The experimental rabbits were fitted with springs exerting 20-g reciprocal force on the maxillary incisors and PGE2 (10 microg/mL) was administered by the intravenous, submucosal, or intraligamentous route after appliance insertion and on days 1, 3, 7, and 14 thereafter. All rabbits were sacrificed on day 21 and their premaxillae were resected for histologic evaluation.
RESULTS
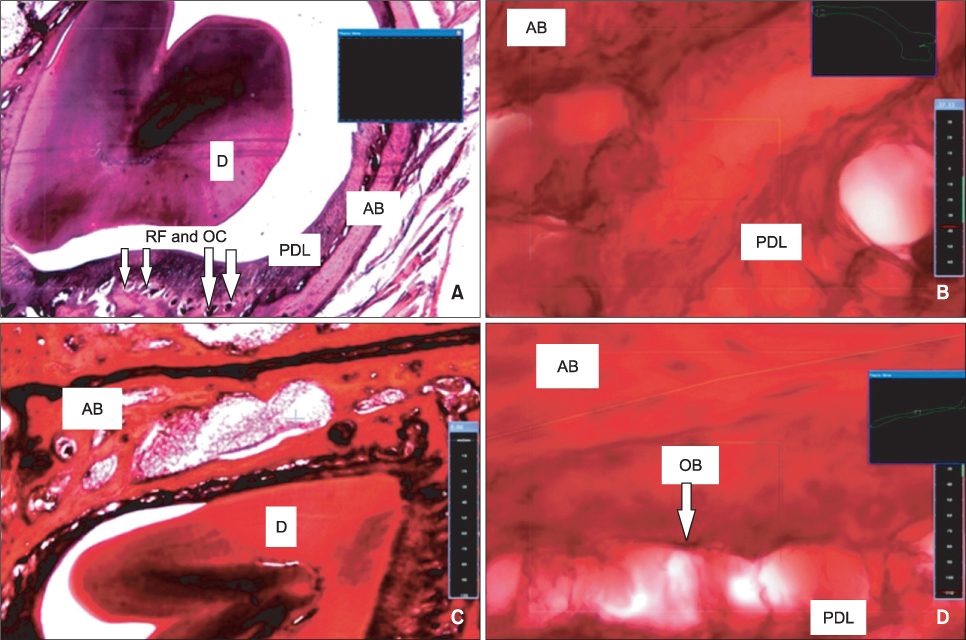
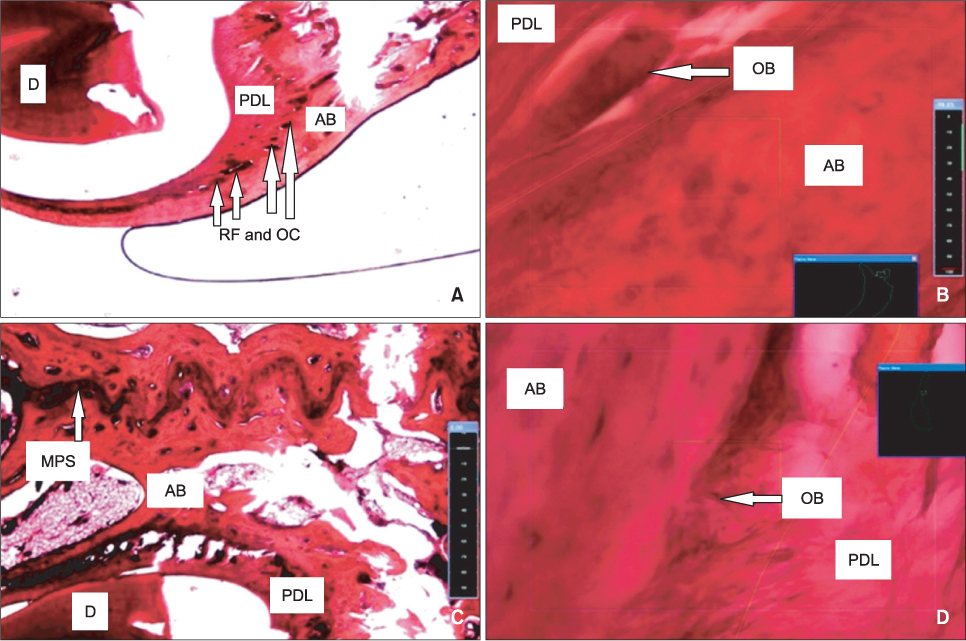
Tooth movement was observed in the experimental and positive control groups, but the intraligamentous PGE2 group had the highest values of all analyzed parameters, including serum calcium and phosphorus levels and osteoclastic and osteoblastic populations (p < 0.001).
CONCLUSIONS
Submucosal and intraligamentous PGE2 administration significantly increases orthodontic tooth movement and bone metabolism, but the intraligamentous route seems to be more effective.
MeSH Terms
Figure
Reference
-
1. van de Velde JP, Kuitert RB, van Ginkel FC, Prahl-Andersen B. Histologic reactions in gingival and alveolar tissues during tooth movement in rabbits. Eur J Orthod. 1988. 10:296–308.
Article2. Persson M. A 100th anniversary: Sandstedt's experiments on tissue changes during tooth movement. J Orthod. 2005. 32:27–28.
Article3. Baumrind S, Buck DL. Rate changes in cell replication and protein synthesis in the periodontal ligament incident to tooth movement. Am J Orthod. 1970. 57:109–131.
Article4. Lilja E, Lindskog S, Hammarström L. Histochemistry of enzymes associated with tissue degradation incident to orthodontic tooth movement. Am J Orthod. 1983. 83:62–75.
Article5. Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984. 85:508–518.
Article6. Yamasaki K, Shibata Y, Fukuhara T. The effect of prostaglandins on experimental tooth movement in monkeys (Macaca fuscata). J Dent Res. 1982. 61:1444–1446.
Article7. Vandevska-Radunovic V, Kvinnsland S, Kvinnsland IH. Effect of experimental tooth movement on nerve fibres immunoreactive to calcitonin gene-related peptide, protein gene product 9.5, and blood vessel density and distribution in rats. Eur J Orthod. 1997. 19:517–529.
Article8. Davidovitch Z, Nicolay OF, Ngan PW, Shanfeld JL. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent Clin North Am. 1988. 32:411–435.9. Mohammed AH, Tatakis DN, Dziak R. Leukotrienes in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1989. 95:231–237.
Article10. Storey E. The nature of tooth movement. Am J Orthod. 1973. 63:292–314.
Article11. Akin E, Gurton AU, Olmez H. Effects of nitric oxide in orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2004. 126:608–614.
Article12. Murray RK, Granner DK, Mayes PA, Rodwell VW. Chapter 16. Physiological İmportance of Lipids. Harper's Biochemistry. 1993. İzmir: BarışYayınevi.13. Leiker BJ, Nanda RS, Currier GF, Howes RI, Sinha PK. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 1995. 108:380–388.
Article14. Seifi M, Eslami B, Saffar AS. The effect of prostaglandin E2 and calcium gluconate on ortho dontic tooth movement and root resorption in rats. Eur J Orthod. 2003. 25:199–204.
Article15. Reich KM, McAllister TN, Gudi S, Frangos JA. Activation of G proteins mediates flow-induced prosta glan din E2 production in osteoblasts. Endocrinology. 1997. 138:1014–1018.
Article16. Yang RS, Fu WM, Wang SM, Lu KS, Liu TK, Lin-Shiau SY. Morphological changes induced by prostaglandin E in cultured rat osteoblasts. Bone. 1998. 22:629–636.
Article17. Kanzaki H, Chiba M, Shimizu Y, Mitani H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res. 2002. 17:210–220.
Article18. Collins MK, Sinclair PM. The local use of vitamin D to increase the rate of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1988. 94:278–284.
Article19. Soma S, Matsumoto S, Higuchi Y, Takano-Yamamoto T, Yamashita K, Kurisu K, et al. Local and chronic application of PTH accelerates tooth movement in rats. J Dent Res. 2000. 79:1717–1724.
Article20. Ashcraft MB, Southard KA, Tolley EA. The effect of corticosteroid-induced osteoporosis on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1992. 102:310–319.
Article21. Saito M, Saito S, Ngan PW, Shanfeld J, Davidovitch Z. Interleukin 1 beta and prostaglandin E are involved in the response of periodontal cells to mechanical stress in vivo and in vitro. Am J Orthod Dentofacial Orthop. 1991. 99:226–240.
Article22. Boekenoogen DI, Sinha PK, Nanda RS, Ghosh J, Currier GF, Howes RI. The effects of exogenous prostaglandin E2 on root resorption in rats. Am J Orthod Dentofacial Orthop. 1996. 109:277–286.
Article23. Brudvik P, Rygh P. Root resorption aft er local injection of prostaglandin E2 during experimental tooth move-ment. Eur J Orthod. 1991. 13:255–263.
Article24. Lee WC. Experimental study of the effect of prostaglandin administration on tooth movement--with particular emphasis on the relationship to the method of PGE1 administration. Am J Orthod Dentofacial Orthop. 1990. 98:231–241.
Article25. Roahen JO, Marshall FJ. The effects of periodontal ligament injection on pulpal and periodontal tissues. J Endod. 1990. 16:28–33.
Article26. Tagger E, Tagger M, Sarnat H, Mass E. Periodontal ligament injection in the dog primary dentition: spread of local anaesthetic solution. Int J Paediatr Dent. 1994. 4:159–166.
Article27. Fuhs QM, Walker WA 3rd, Gough RW, Schindler WG, Hartman KS. The periodontal ligament injection: histological effects on the periodontium in dogs. J Endod. 1983. 9:411–415.
Article28. Odacı E, Yıldırım Ş, Bahadır A, Canan S, Şahin B, Baş O, et al. The possible error sources of new stereological methods and their solutions. Turkiye Klinikleri J Med Sci. 2004. 24:78–87.29. Graber TM. Orthodontics: Current Principles and Techniques. 2000. 3rd ed. St. Louis: Mosby;193–257.30. Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod. 1967. 53:721–745.
Article31. Ersöz M. The changes in the hormones playing a role in the bone resorption and deposition during orthodontic tooth movement [doctorate Tesis]. 2004. Erzurum: Atatürk University.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An experimental study on the effect of prostaglandin E2 on alveolar bone resorption induced by tooth movement in rats
- Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption
- A study on the effect of prostaglandin E2 on tooth movement and root resorption in cats
- Effect of 125 Hz and 150 Hz vibrational frequency electric toothbrushes on the rate of orthodontic tooth movement and prostaglandin E2 levels
- Corticotomy and the Intrusive Tooth Movement