Korean J Radiol.
2011 Oct;12(5):602-610. 10.3348/kjr.2011.12.5.602.
Evaluation of Antiangiogenic Effects of a New Synthetic Candidate Drug KR-31831 on Xenografted Ovarian Carcinoma Using Dynamic Contrast Enhanced MRI
- Affiliations
-
- 1Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. drpjeon@gmail.com
- 2Center for Molecular and Cellular Imaging, Samsung Biomedical Research Institute, Samsung Medical Center, Seoul 135-710, Korea.
- KMID: 1116446
- DOI: http://doi.org/10.3348/kjr.2011.12.5.602
Abstract
OBJECTIVE
The purpose of this research was to investigate the anti-angiogenic inhibitory effect of KR-31831, a newly developed anti-angiogenic agent, on an in vivo human ovarian carcinoma model using dynamic contrast-enhanced (DCE) MRI.
MATERIALS AND METHODS
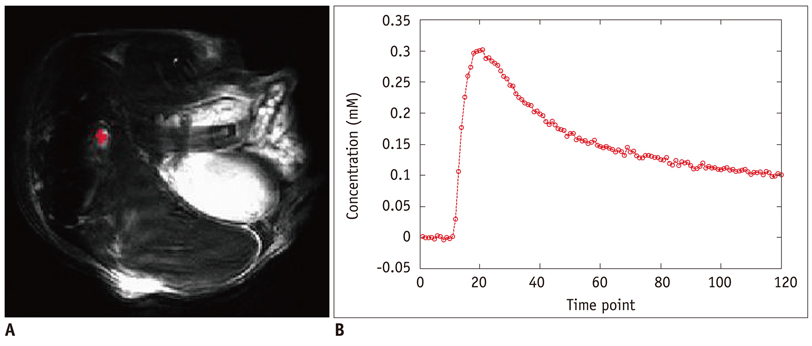
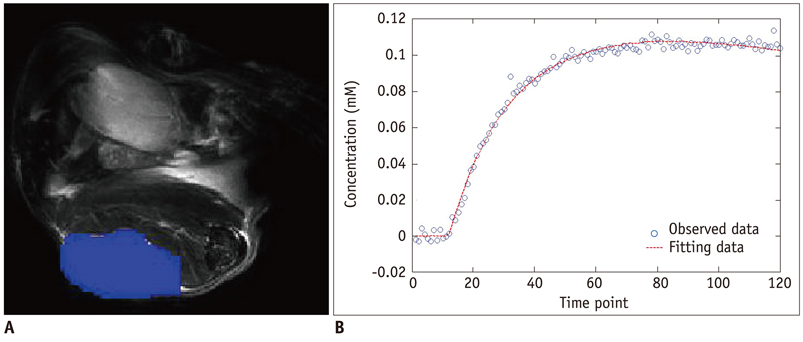
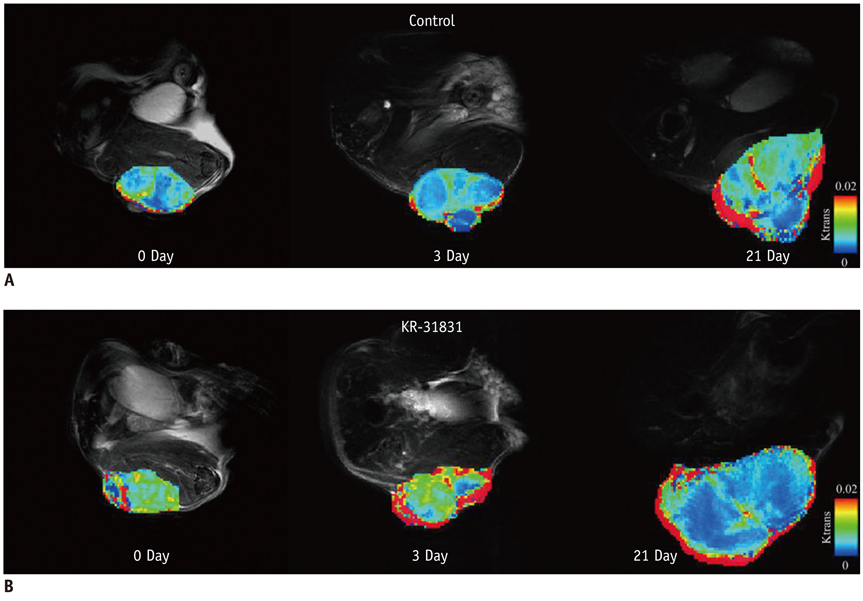
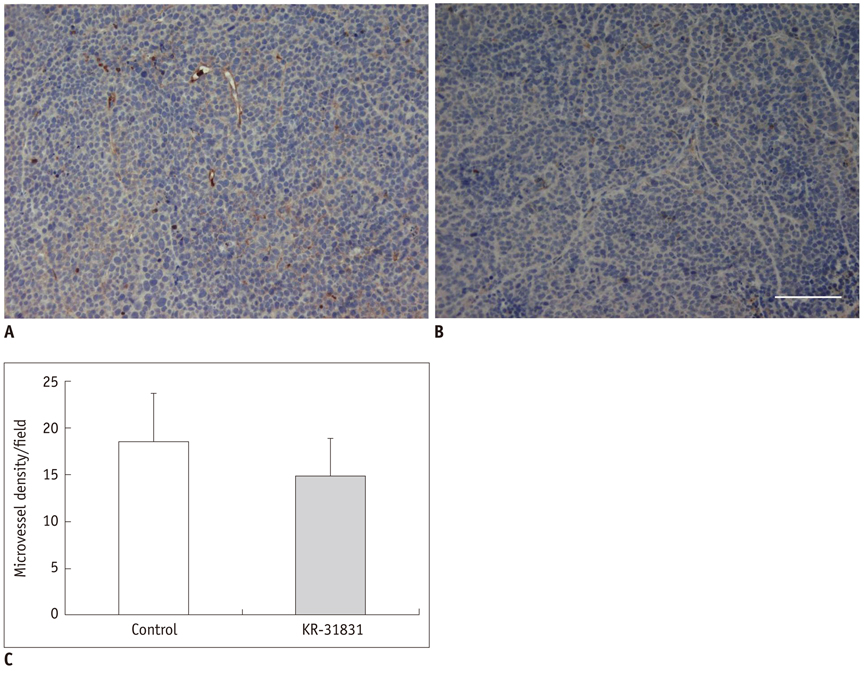
Xenografted ovarian tumors were established by subcutaneous injection of SKOV3 cells into mice. The mice were treated daily with KR-31831 at 50 mg/kg for 21 days. Tumor tissues were excised corresponding to the DCE-MRI sections for evaluation of MVD with CD31 immunohistochemistry. All in vivo MRIs were performed on a 7.0 Tesla micro-MRI System. DCE-MRI was acquired prior to initiating treatment with KR-31831 and again on days 3 and 21 after treatment. The permeability parameters (Ktrans, ve, and vp) were estimated using a pharmacokinetic model.
RESULTS
Qualitatively, the Ktrans parametric mapping showed different changes before and after treatment with KR-31831 in the treatment group. For quantification of this change, the median of Ktrans values were compared before and after treatments in the control and KR-31831-treated groups. A non-parametric statistical test (Wilcoxon signed-rank test) showed decreasing Ktrans values on day 21 compared to days 0 and 3 in the KR-31831-treated group (p < 0.05), whereas there was no significant difference in the control group (p = 0.84).
CONCLUSION
Our results suggest that DCE-MRI can be a useful tool by which to evaluate the anti-angiogenic effect of KR-31831 on a xenografted human ovarian carcinoma model.
MeSH Terms
-
Angiogenesis Inhibitors/*pharmacology
Animals
Benzopyrans/*pharmacology
Cell Line, Tumor
*Contrast Media
Female
Humans
Imidazoles/*pharmacology
Immunohistochemistry
*Magnetic Resonance Imaging
Mice
Mice, Inbred BALB C
Mice, Nude
Microvessels/pathology
Neoplasm Transplantation
Ovarian Neoplasms/*blood supply/pathology
Figure
Reference
-
1. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990. 82:4–6.2. Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997. 390:404–407.3. Ko EY, Lee SH, Kim HH, Kim SM, Shin MJ, Kim N, et al. Evaluation of tumor angiogenesis with a second-generation US contrast medium in a rat breast tumor model. Korean J Radiol. 2008. 9:243–249.4. Conn G, Bayne ML, Soderman DD, Kwok PW, Sullivan KA, Palisi TM, et al. Amino acid and cDNA sequences of a vascular endothelial cell mitogen that is homologous to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1990. 87:2628–2632.5. Fox SB, Gasparini G, Harris AL. Angiogenesis: pathological, prognostic, and growth-factor pathways and their link to trial design and anticancer drugs. Lancet Oncol. 2001. 2:278–289.6. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997. 18:4–25.7. Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989. 161:851–858.8. Plouet J, Schilling J, Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989. 8:3801–3806.9. Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000. 60:2178–2189.10. Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995. 146:1029–1039.11. Prewett M, Huber J, Li Y, Santiago A, O'Connor W, King K, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999. 59:5209–5218.12. Kozin SV, Boucher Y, Hicklin DJ, Bohlen P, Jain RK, Suit HD. Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res. 2001. 61:39–44.13. Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998. 153:1249–1256.14. Park SY, Seo EH, Song HS, Jung SY, Lee YK, Yi KY, et al. KR-31831, benzopyran derivative, inhibits VEGF-induced angiogenesis of HUVECs through suppressing KDR expression. Int J Oncol. 2008. 32:1311–1315.15. Yi EY, Park SY, Song HS, Son MJ, Yi KY, Yoo SE, et al. KR-31831, a new synthetic anti-ischemic agent, inhibits in vivo and in vitro angiogenesis. Exp Mol Med. 2006. 38:502–508.16. Kim HH, Paek IB, Ji HY, Lee S, Yi KY, Lee HS. Metabolism of a novel antiangiogenic agent KR-31831 in rats using liquid chromatography-electrospray mass spectrometry. J Sep Sci. 2005. 28:1818–1822.17. Kim SJ, Lee HI, Ji HY, Moon Y, Paek IB, Lee S, et al. Pharmacokinetics of a novel antiangiogenic agent KR-31831 in rats. Biopharm Drug Dispos. 2005. 26:21–26.18. O'Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007. 96:189–195.19. Zahra MA, Hollingsworth KG, Sala E, Lomas DJ, Tan LT. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007. 8:63–74.20. Pedersen M, Morkenborg J, Jensen FT, Stodkilde-Jorgensen H, Djurhuus JC, Frokiaer J. In vivo measurements of relaxivities in the rat kidney cortex. J Magn Reson Imaging. 2000. 12:289–296.21. Deoni SC, Rutt BK, Peters TM. Rapid combined T1 and T2 mapping using gradient recalled acquisition in the steady state. Magn Reson Med. 2003. 49:515–526.22. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999. 10:223–232.23. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996. 29:162–173.24. Shinriki S, Jono H, Ota K, Ueda M, Kudo M, Ota T, et al. Humanized anti-interleukin-6 receptor antibody suppresses tumor angiogenesis and in vivo growth of human oral squamous cell carcinoma. Clin Cancer Res. 2009. 15:5426–5434.25. Pandya NM, Dhalla NS, Santani DD. Angiogenesis--a new target for future therapy. Vascul Pharmacol. 2006. 44:265–274.26. Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997. 57:4593–4599.27. Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, et al. Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996. 56:3540–3545.28. Jun HY, Yin HH, Kim SH, Park SH, Kim HS, Yoon KH. Visualization of tumor angiogenesis using MR imaging contrast agent Gd-DTPA-anti-VEGF receptor 2 antibody conjugate in a mouse tumor model. Korean J Radiol. 2010. 11:449–456.29. Xiong HQ, Herbst R, Faria SC, Scholz C, Davis D, Jackson EF, et al. A phase I surrogate endpoint study of SU6668 in patients with solid tumors. Invest New Drugs. 2004. 22:459–466.30. Thomassin-Naggara I, Bazot M, Darai E, Callard P, Thomassin J, Cuenod CA. Epithelial ovarian tumors: value of dynamic contrast-enhanced MR imaging and correlation with tumor angiogenesis. Radiology. 2008. 248:148–159.31. Hillman GG, Singh-Gupta V, Zhang H, Al-Bashir AK, Katkuri Y, Li M, et al. Dynamic contrast-enhanced magnetic resonance imaging of vascular changes induced by sunitinib in papillary renal cell carcinoma xenograft tumors. Neoplasia. 2009. 11:910–920.32. Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991. 17:357–367.33. Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991. 15:621–628.34. Larsson HB, Stubgaard M, Frederiksen JL, Jensen M, Henriksen O, Paulson OB. Quantitation of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn Reson Med. 1990. 16:117–131.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of T1 Map Information Based on Synthetic MRI for Dynamic Contrast-Enhanced Imaging: A Comparison Study with the Fixed Baseline T1 Value Method
- KR-31831, a new synthetic anti-ischemic agent, inhibits in vivo and in vitro angiogenesis
- Current Limitations and Potential Breakthroughs for the Early Diagnosis of Hepatocellular Carcinoma
- Dynamic Contrast-Enhanced MRI and Its Applications in Various Central Nervous System Diseases
- Dynamic Contrast-Enhanced MRI for Monitoring Antiangiogenic Treatment: Determination of Accurate and Reliable Perfusion Parameters in a Longitudinal Study of a Mouse Xenograft Model