Korean J Radiol.
2013 Aug;14(4):589-596. 10.3348/kjr.2013.14.4.589.
Dynamic Contrast-Enhanced MRI for Monitoring Antiangiogenic Treatment: Determination of Accurate and Reliable Perfusion Parameters in a Longitudinal Study of a Mouse Xenograft Model
- Affiliations
-
- 1Division of Magnetic Resonance, Korea Basic Science Institute, Cheongwon 363-883, Korea. kim.jeongkon@gmail.com
- 2Department of Radiology, Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- 3Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA 02129, USA.
- 4Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- KMID: 1715763
- DOI: http://doi.org/10.3348/kjr.2013.14.4.589
Abstract
OBJECTIVE
To determine the reliable perfusion parameters in dynamic contrast-enhanced MRI (DCE-MRI) for the monitoring antiangiogenic treatment in mice.
MATERIALS AND METHODS
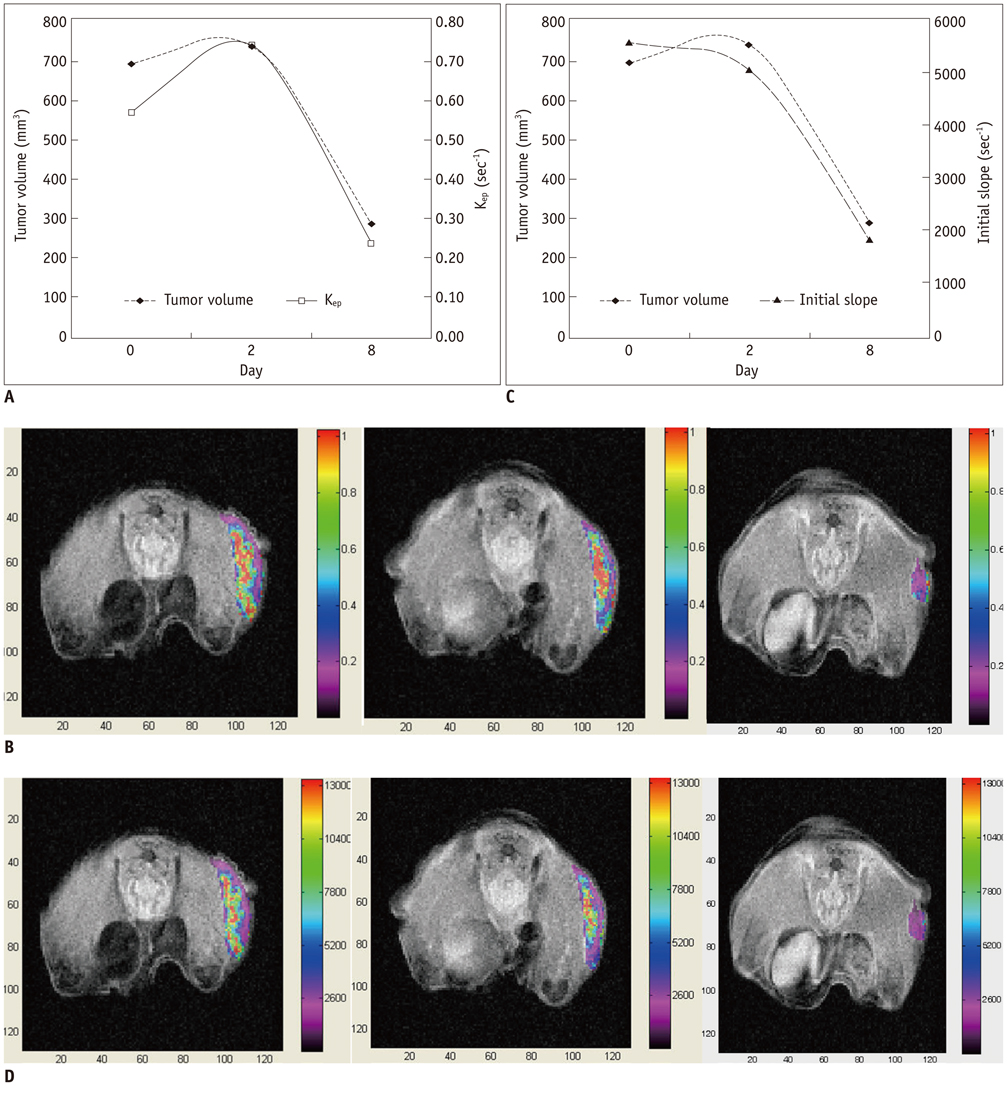
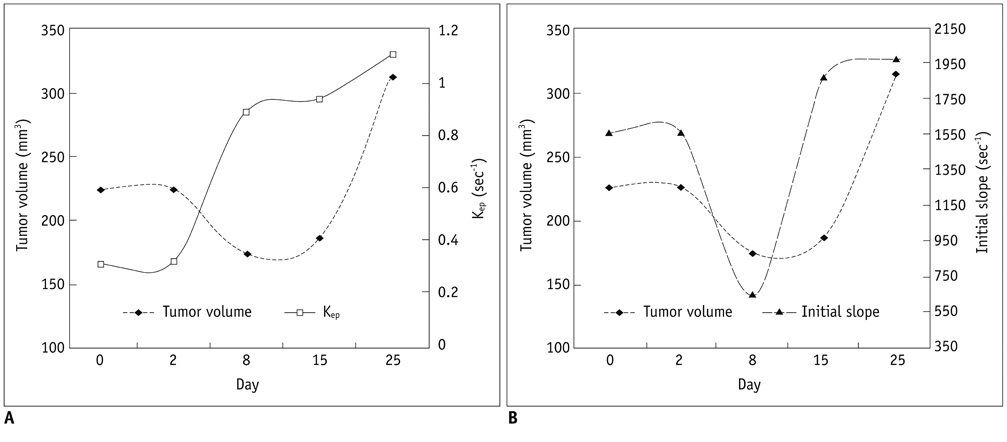
Mice, with U-118 MG tumor, were treated with either saline (n = 3) or antiangiogenic agent (sunitinib, n = 8). Before (day 0) and after (days 2, 8, 15, 25) treatment, DCE examinations using correlations of perfusion parameters (Kep, Kel, and AH from two compartment model; time to peak, initial slope and % enhancement from time-intensity curve analysis) were evaluated.
RESULTS
Tumor growth rate was found to be 129% +/- 28 in control group, -33% +/- 11 in four mice with sunitinib-treatment (tumor regression) and 47% +/- 15 in four with sunitinib-treatment (growth retardation). Kep (r = 0.80) and initial slope (r = 0.84) showed strong positive correlation to the initial tumor volume (p < 0.05). In control mice, tumor regression group and growth retardation group animals, Kep (r : 0.75, 0.78, 0.81, 0.69) and initial slope (r : 0.79, 0.65, 0.67, 0.84) showed significant correlation with tumor volume (p < 0.01). In four mice with tumor re-growth, Kep and initial slope increased 20% or greater at earlier (n = 2) than or same periods (n = 2) to when the tumor started to re-grow with 20% or greater growth rate.
CONCLUSION
Kep and initial slope may a reliable parameters for monitoring the response of antiangiogenic treatment.
MeSH Terms
-
Angiogenesis Inhibitors/therapeutic use
Animals
Contrast Media/*diagnostic use
Female
Heterografts
Indoles/*therapeutic use
Longitudinal Studies
Magnetic Resonance Imaging/*methods
Mice
Mice, Inbred BALB C
Neoplasm Transplantation
Neoplasms, Experimental/*diagnosis/drug therapy/pathology
Pyrroles/*therapeutic use
Reproducibility of Results
Tumor Burden
Angiogenesis Inhibitors
Contrast Media
Indoles
Pyrroles
Figure
Reference
-
1. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008; 358:2039–2049.2. Gossmann A, Helbich TH, Kuriyama N, Ostrowitzki S, Roberts TP, Shames DM, et al. Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging. 2002; 15:233–240.3. Maxwell RJ, Wilson J, Prise VE, Vojnovic B, Rustin GJ, Lodge MA, et al. Evaluation of the anti-vascular effects of combretastatin in rodent tumours by dynamic contrast enhanced MRI. NMR Biomed. 2002; 15:89–98.4. Rosen MA, Schnall MD. Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma. Clin Cancer Res. 2007; 13(2 Pt 2):770s–776s.5. Ferl GZ, Port RE. Quantification of antiangiogenic and antivascular drug activity by kinetic analysis of DCE-MRI data. Clin Pharmacol Ther. 2012; 92:118–124.6. Potapova O, Laird AD, Nannini MA, Barone A, Li G, Moss KG, et al. Contribution of individual targets to the antitumor efficacy of the multitargeted receptor tyrosine kinase inhibitor SU11248. Mol Cancer Ther. 2006; 5:1280–1289.7. Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003; 9:327–337.8. Hoffmann U, Brix G, Knopp MV, Hess T, Lorenz WJ. Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med. 1995; 33:506–514.9. Buckley DL, Kerslake RW, Blackband SJ, Horsman A. Quantitative analysis of multi-slice Gd-DTPA enhanced dynamic MR images using an automated simplex minimization procedure. Magn Reson Med. 1994; 32:646–651.10. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997; 7:91–101.11. Zou KH, Tuncali K, Silverman SG. Correlation and simple linear regression. Radiology. 2003; 227:617–622.12. Muruganandham M, Lupu M, Dyke JP, Matei C, Linn M, Packman K, et al. Preclinical evaluation of tumor microvascular response to a novel antiangiogenic/antitumor agent RO0281501 by dynamic contrast-enhanced MRI at 1.5 T. Mol Cancer Ther. 2006; 5:1950–1957.13. Pickles MD, Manton DJ, Lowry M, Turnbull LW. Prognostic value of pre-treatment DCE-MRI parameters in predicting disease free and overall survival for breast cancer patients undergoing neoadjuvant chemotherapy. Eur J Radiol. 2009; 71:498–505.14. Elsayed YA, Sausville EA. Selected novel anticancer treatments targeting cell signaling proteins. Oncologist. 2001; 6:517–537.15. Morgan B, Utting JF, Higginson A, Thomas AL, Steward WP, Horsfield MA. A simple, reproducible method for monitoring the treatment of tumours using dynamic contrast-enhanced MR imaging. Br J Cancer. 2006; 94:1420–1427.16. Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991; 15:621–628.17. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.18. Evelhoch J, Garwood M, Vigneron D, Knopp M, Sullivan D, Menkens A, et al. Expanding the use of magnetic resonance in the assessment of tumor response to therapy: workshop report. Cancer Res. 2005; 65:7041–7044.19. de Lussanet QG, Langereis S, Beets-Tan RG, van Genderen MH, Griffioen AW, van Engelshoven JM, et al. Dynamic contrast-enhanced MR imaging kinetic parameters and molecular weight of dendritic contrast agents in tumor angiogenesis in mice. Radiology. 2005; 235:65–72.20. Buckley DL, Drew PJ, Mussurakis S, Monson JR, Horsman A. Microvessel density of invasive breast cancer assessed by dynamic Gd-DTPA enhanced MRI. J Magn Reson Imaging. 1997; 7:461–464.21. Cooper RA, Carrington BM, Loncaster JA, Todd SM, Davidson SE, Logue JP, et al. Tumour oxygenation levels correlate with dynamic contrast-enhanced magnetic resonance imaging parameters in carcinoma of the cervix. Radiother Oncol. 2000; 57:53–59.22. Ahn SJ, An CS, Koom WS, Song HT, Suh JS. Correlations of 3T DCE-MRI quantitative parameters with microvessel density in a human-colorectal-cancer xenograft mouse model. Korean J Radiol. 2011; 12:722–730.23. Kang H, Lee HY, Lee KS, Kim JH. Imaging-based tumor treatment response evaluation: review of conventional, new, and emerging concepts. Korean J Radiol. 2012; 13:371–390.24. Kim JK, Jang YJ, Cho G. Multidisciplinary functional MR imaging for prostate cancer. Korean J Radiol. 2009; 10:535–551.25. Knopp MV, Weiss E, Sinn HP, Mattern J, Junkermann H, Radeleff J, et al. Pathophysiologic basis of contrast enhancement in breast tumors. J Magn Reson Imaging. 1999; 10:260–266.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlations of 3T DCE-MRI Quantitative Parameters with Microvessel Density in a Human-Colorectal-Cancer Xenograft Mouse Model
- Quantitative Assessment of Tumor Responses after Radiation Therapy in a DLD-1 Colon Cancer Mouse Model Using Serial Dynamic Contrast-Enhanced Magnetic Resonance Imaging
- Application of T1 Map Information Based on Synthetic MRI for Dynamic Contrast-Enhanced Imaging: A Comparison Study with the Fixed Baseline T1 Value Method
- Usefulness of a Pharmacokinetic Model Based on Dynamic Contrast-enhanced MRI for the Detection and Localization of Prostate Cancer
- Permeability Parameters Measured with Dynamic Contrast-Enhanced MRI: Correlation with the Extravasation of Evans Blue in a Rat Model of Transient Cerebral Ischemia