Korean J Radiol.
2015 Aug;16(4):791-797. 10.3348/kjr.2015.16.4.791.
Permeability Parameters Measured with Dynamic Contrast-Enhanced MRI: Correlation with the Extravasation of Evans Blue in a Rat Model of Transient Cerebral Ischemia
- Affiliations
-
- 1Department of Radiology, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 2Department of Radiology, College of Medicine, Yonsei University, Seoul 120-752, Korea. slee@yuhs.ac
- 3Department of Radiology, College of Medicine, Seoul National University, Seoul 110-744, Korea.
- 4Department of Anatomy, College of Medicine, Yonsei University, Seoul 120-752, Korea.
- 5Department of Neurology, College of Medicine, Yonsei University, Seoul 120-752, Korea.
- KMID: 2155552
- DOI: http://doi.org/10.3348/kjr.2015.16.4.791
Abstract
OBJECTIVE
The purpose of this study was to correlate permeability parameters measured with dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) using a clinical 3-tesla scanner with extravasation of Evans blue in a rat model with transient cerebral ischemia.
MATERIALS AND METHODS
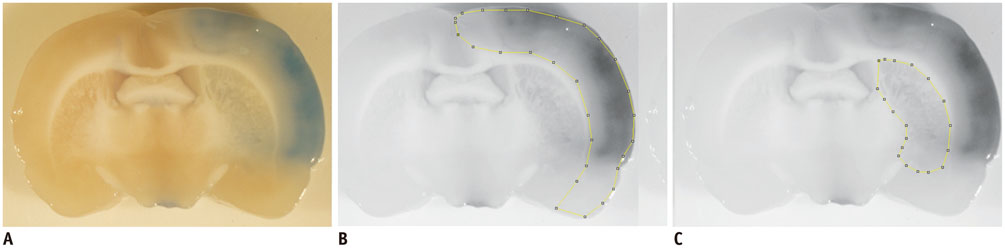
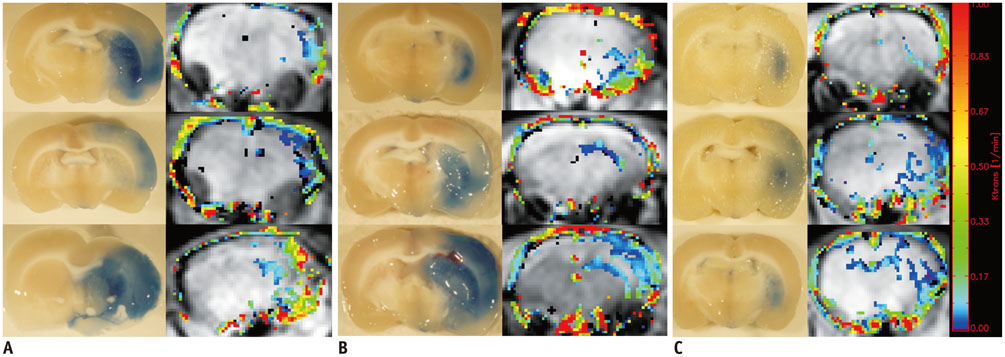
Sprague-Dawley rats (n = 13) with transient middle cerebral artery occlusion were imaged using a 3-tesla MRI with an 8-channel wrist coil. DCE-MRI was performed 12 hours, 18 hours, and 36 hours after reperfusion. Permeability parameters (K(trans), v(e), and v(p)) from DCE-MRI were calculated. Evans blue was injected after DCE-MRI and extravasation of Evans blue was correlated as a reference with the integrity of the blood-brain barrier. Correlation analysis was performed between permeability parameters and the extravasation of Evans blue.
RESULTS
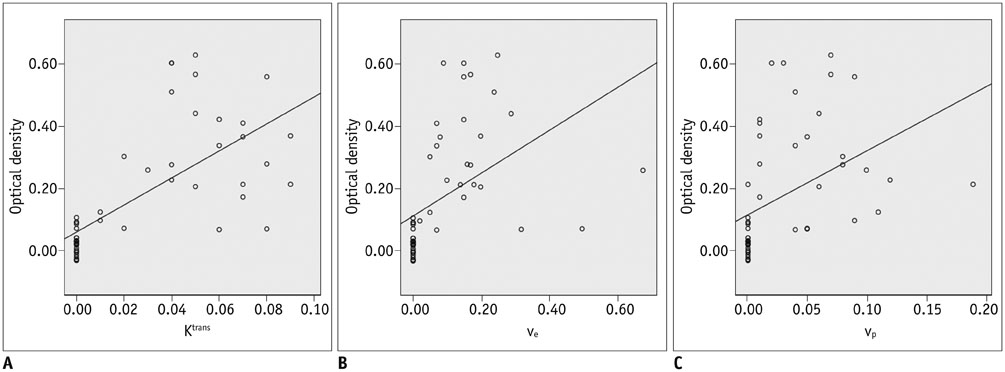
All permeability parameters (K(trans), v(e), and v(p)) showed a linear correlation with extravasation of Evans blue. Among them, K(trans) showed highest values of both the correlation coefficient and the coefficient of determination (0.687 and 0.473 respectively, p < 0.001).
CONCLUSION
Permeability parameters obtained by DCE-MRI at 3-T are well-correlated with Evans blue extravasation, and K(trans) shows the strongest correlation among the tested parameters.
Keyword
MeSH Terms
Figure
Reference
-
1. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587.2. Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis. 2007; 24:1–10.3. Tanne D, Kasner SE, Demchuk AM, Koren-Morag N, Hanson S, Grond M, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation. 2002; 105:1679–1685.4. The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997; 28:2109–2118.5. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000; 355:1670–1674.6. Kase CS, Furlan AJ, Wechsler LR, Higashida RT, Rowley HA, Hart RG, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001; 57:1603–1610.7. Larrue V, von Kummer RR, Müller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke. 2001; 32:438–441.8. Lansberg MG, Thijs VN, Bammer R, Kemp S, Wijman CA, Marks MP, et al. Risk factors of symptomatic intracerebral hemorrhage after tPA therapy for acute stroke. Stroke. 2007; 38:2275–2278.9. Alsop DC, Makovetskaya E, Kumar S, Selim M, Schlaug G. Markedly reduced apparent blood volume on bolus contrast magnetic resonance imaging as a predictor of hemorrhage after thrombolytic therapy for acute ischemic stroke. Stroke. 2005; 36:746–750.10. Kim EY, Na DG, Kim SS, Lee KH, Ryoo JW, Kim HK. Prediction of hemorrhagic transformation in acute ischemic stroke: role of diffusion-weighted imaging and early parenchymal enhancement. AJNR Am J Neuroradiol. 2005; 26:1050–1055.11. Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005; 36:66–73.12. Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE). Stroke. 2008; 39:2257–2263.13. Bang OY, Saver JL, Alger JR, Shah SH, Buck BH, Starkman S, et al. Patterns and predictors of blood-brain barrier permeability derangements in acute ischemic stroke. Stroke. 2009; 40:454–461.14. Selim M, Fink JN, Kumar S, Caplan LR, Horkan C, Chen Y, et al. Predictors of hemorrhagic transformation after intravenous recombinant tissue plasminogen activator: prognostic value of the initial apparent diffusion coefficient and diffusion-weighted lesion volume. Stroke. 2002; 33:2047–2052.15. Lin K, Kazmi KS, Law M, Babb J, Peccerelli N, Pramanik BK. Measuring elevated microvascular permeability and predicting hemorrhagic transformation in acute ischemic stroke using first-pass dynamic perfusion CT imaging. AJNR Am J Neuroradiol. 2007; 28:1292–1298.16. Hom J, Dankbaar JW, Soares BP, Schneider T, Cheng SC, Bredno J, et al. Blood-brain barrier permeability assessed by perfusion CT predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR Am J Neuroradiol. 2011; 32:41–48.17. Lin K. Predicting transformation to type 2 parenchymal hematoma in acute ischemic stroke by CT permeability imaging. AJNR Am J Neuroradiol. 2011; 32:E124. author reply E125.18. Kassner A, Roberts T, Taylor K, Silver F, Mikulis D. Prediction of hemorrhage in acute ischemic stroke using permeability MR imaging. AJNR Am J Neuroradiol. 2005; 26:2213–2217.19. Kassner A, Roberts TP, Moran B, Silver FL, Mikulis DJ. Recombinant tissue plasminogen activator increases blood-brain barrier disruption in acute ischemic stroke: an MR imaging permeability study. AJNR Am J Neuroradiol. 2009; 30:1864–1869.20. Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood-brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 tesla. Magn Reson Med. 2009; 62:1270–1281.21. Thornhill RE, Chen S, Rammo W, Mikulis DJ, Kassner A. Contrast-enhanced MR imaging in acute ischemic stroke: T2* measures of blood-brain barrier permeability and their relationship to T1 estimates and hemorrhagic transformation. AJNR Am J Neuroradiol. 2010; 31:1015–1022.22. Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004; 35:11 Suppl 1. 2659–2661.23. Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008; 32:200–219.24. Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol. 2007; 83:140–148.25. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999; 22:391–397.26. Ahn SK, Hong S, Park YM, Lee WT, Park KA, Lee JE. Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res. 2011; 1373:48–54.27. Kassner A, Mandell DM, Mikulis DJ. Measuring permeability in acute ischemic stroke. Neuroimaging Clin N Am. 2011; 21:315–325. x–xi.28. Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007; 38:1044–1049.29. Belayev L, Busto R, Ikeda M, Rubin LL, Kajiwara A, Morgan L, et al. Protection against blood-brain barrier disruption in focal cerebral ischemia by the type IV phosphodiesterase inhibitor BBB022: a quantitative study. Brain Res. 1998; 787:277–285.30. Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, et al. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008; 153:175–181.31. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.32. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–675.33. Durukan A, Marinkovic I, Strbian D, Pitkonen M, Pedrono E, Soinne L, et al. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res. 2009; 1280:158–165.34. Jiang Q, Ewing JR, Ding GL, Zhang L, Zhang ZG, Li L, et al. Quantitative evaluation of BBB permeability after embolic stroke in rat using MRI. J Cereb Blood Flow Metab. 2005; 25:583–592.35. Ding G, Jiang Q, Li L, Zhang L, Gang Zhang Z, Ledbetter KA, et al. Detection of BBB disruption and hemorrhage by Gd-DTPA enhanced MRI after embolic stroke in rat. Brain Res. 2006; 1114:195–203.36. Taheri S, Candelario-Jalil E, Estrada EY, Rosenberg GA. Spatiotemporal correlations between blood-brain barrier permeability and apparent diffusion coefficient in a rat model of ischemic stroke. PLoS One. 2009; 4:e6597.37. Neumann-Haefelin C, Brinker G, Uhlenküken U, Pillekamp F, Hossmann KA, Hoehn M. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: an MRI investigation in rat brain. Stroke. 2002; 33:1392–1398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neurogenic Inflammation Induced by Hypertonic Saline in Rat Nasal Mucosa
- Assessment of Blood-Brain Barrier Permeability by Dynamic Contrast-Enhanced MRI in Transient Middle Cerebral Artery Occlusion Model after Localized Brain Cooling in Rats
- Glioma Grading Capability: Comparisons among Parameters from Dynamic Contrast-Enhanced MRI and ADC Value on DWI
- Increased vascular permeability and reduced neutral endopeptidase activity in rat nasal mucosa after ozone exposure
- Effect of Endogenous Nitric Oxide on Neurogenic Plasma Extravasation in Nasal Mucosa of Rat