J Korean Med Sci.
2008 Dec;23(6):1033-1038. 10.3346/jkms.2008.23.6.1033.
Additive Antinociception between Intrathecal Sildenafil and Morphine in the Rat Formalin Test
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chonnam National University, Medical School, Gwangju, Korea. mhyoon@chonnam.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Chosun University, Medical School, Gwangju, Korea.
- 3The Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju, Korea.
- KMID: 1107530
- DOI: http://doi.org/10.3346/jkms.2008.23.6.1033
Abstract
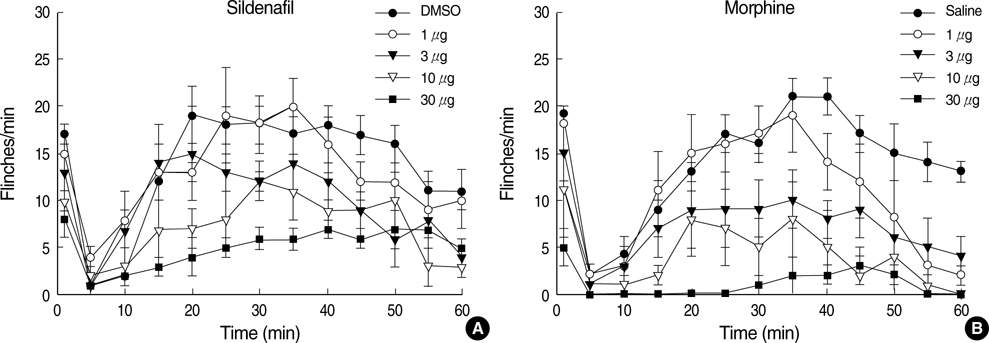
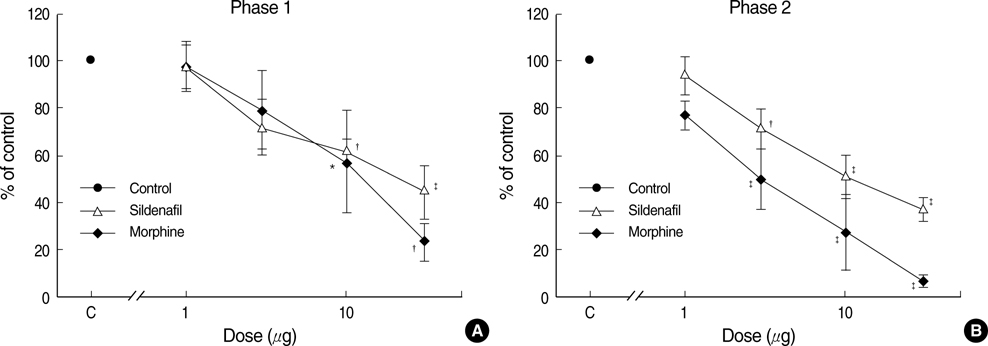
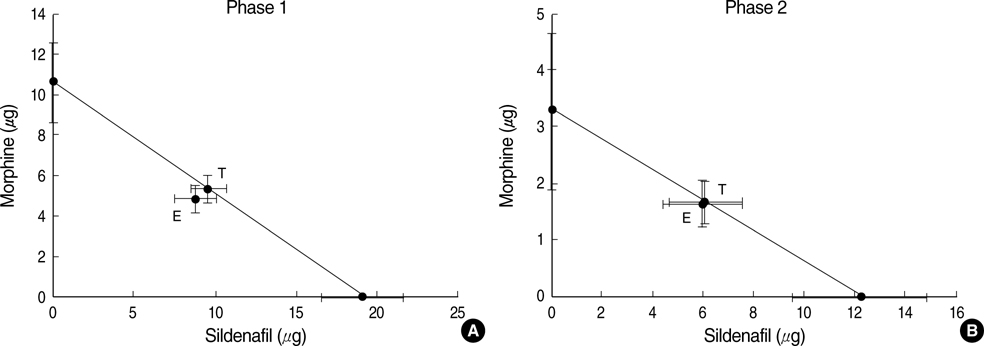
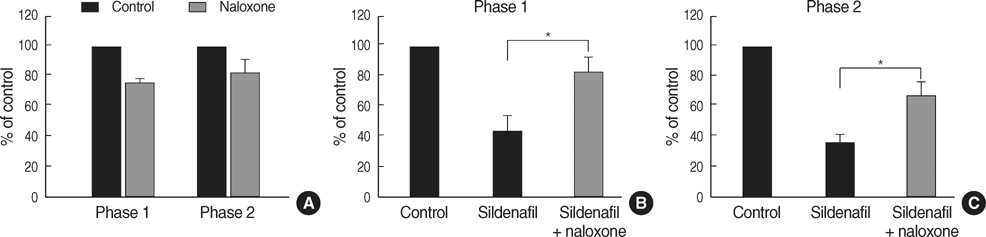
- The possible characteristics of spinal interaction between sildenafil (phosphodiesterase 5 inhibitor) and morphine on formalin-induced nociception in rats was examined. Then the role of the opioid receptor in the effect of sildenafil was further investigated. Catheters were inserted into the intrathecal space of male Sprague-Dawley rats. For induction of pain, 50 microliter of 5% formalin solution was applied to the hindpaw. Isobolographic analysis was used for the evaluation of drug interaction between sildenafil and morphine. Furthermore, naloxone was intrathecally given to verify the involvement of the opioid receptor in the antinociception of sildenafil. Both sildenafil and morphine produced an antinociceptive effect during phase 1 and phase 2 in the formalin test. The isobolographic analysis revealed an additive interaction after intrathecal delivery of the sildenafil-morphine mixture in both phases. Intrathecal naloxone reversed the antinociception of sildenafil in both phases. These results suggest that sildenafil, morphine, and the mixture of the two drugs are effective against acute pain and facilitated pain state at the spinal level. Thus, the spinal combination of sildenafil with morphine may be useful in the management of the same state. Furthermore, the opioid receptor is contributable to the antinocieptive mechanism of sildenafil at the spinal level.
Keyword
MeSH Terms
-
Analgesics/*administration & dosage
Analgesics, Opioid/*administration & dosage
Animals
Behavior, Animal/drug effects
Dose-Response Relationship, Drug
Drug Synergism
Formaldehyde/toxicity
Injections, Spinal
Male
Morphine/*administration & dosage
Naloxone/administration & dosage
Narcotic Antagonists/administration & dosage
Pain/chemically induced/therapy
Pain Measurement/drug effects
Phosphodiesterase Inhibitors/*administration & dosage
Piperazines/*administration & dosage
Purines/administration & dosage
Rats
Rats, Sprague-Dawley
Sulfones/*administration & dosage
Time Factors
Figure
Cited by 2 articles
-
Epidural Dexamethasone Decreased Inflammatory Hyperalgesia and Spinal cPLA2 Expression in a Rat Formalin Test
Sam-Hong Min, Jung-Sub Soh, Ji-Yong Park, Sung-Uk Choi, Hye-Won Lee, Jae-Jin Lee, Jae-Hwan Kim
Yonsei Med J. 2014;55(6):1631-1639. doi: 10.3349/ymj.2014.55.6.1631.Antinociceptive effect of intrathecal sec-O-glucosylhamaudol on the formalin-induced pain in rats
Sang Hun Kim, Hwa Song Jong, Myung Ha Yoon, Seon Hee Oh, Ki Tae Jung
Korean J Pain. 2017;30(2):98-103. doi: 10.3344/kjp.2017.30.2.98.
Reference
-
1. Ferreira J, Santos AR, Calixto JB. The role of systemic, spinal and supraspinal L-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology. 1999. 38:835–842.
Article2. Tao YX, Hassan A, Haddad E, Johns RA. Expression and action of cyclic GMP-dependent protein kinase Ialpha in inflammatory hyperalgesia in rat spinal cord. Neuroscience. 2000. 95:525–533.3. Ferreira SH, Nakamura M. Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979. 18:179–190.4. Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001. 897:9–19.
Article5. Pyne NJ, Arshavsky V, Lochhead A. cGMP signal termination. Biochem Soc Trans. 1996. 24:1019–1022.
Article6. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995. 75:725–748.
Article7. Boolell M, Gepi-Attee S, Gingell JC, Allen MJ. Sildenafil, a novel effective oral therapy for male erectile dysfunction. Br J Urol. 1996. 78:257–261.
Article8. Terrett NK, Bell AS, Brown D, Ellois P. Sildenafil (Viagra™), a potent and selective inhibitor of type 5 cGMP phospodiesterase with utility for the treatment of male erectile dysfunction. Bioorg Med Chem Lett. 1996. 6:1819–1824.9. Patil CS, Singh VP, Kulkarni SK. Peripheral and central activation of nitric oxide-cyclic GMP pathway by sildenafil. Inflammopharmacology. 2005. 13:467–478.
Article10. Araiza-Saldaña CI, Reyes-García G, Bermúdez-Ocaña DY, Francisca Pérez-Severiano F, Granados-Soto V. Effect of diabetes on the mechanisms of intrathecal antinociception of sildenafil in rats. Eur J Pharmacol. 2005. 527:60–70.
Article11. Przesmycki K, Dzieciuch JA, Czuczwar SJ, Kleinrok Z. Isobolographic analysis of interaction between intrathecal morphine and clonidine in the formalin test in rats. Eur J Pharmacol. 1997. 337:11–17.
Article12. Gouardères C, Sutak M, Zajac JM, Jhamandas K. Role of adenosine in the spinal antinociceptive and morphine modulatory actions of neuropeptide FF analogs. Eur J Pharmacol. 2000. 406:391–401.
Article13. Nishiyama T. Interaction between intrathecal morphine and glutamate receptor antagonists in formalin test. Eur J Pharmacol. 2000. 395:203–210.
Article14. Yoon MH, Choi JI, Kim SJ, Kim CM, Bae HB, Chung ST. Synergistic antinociception between zaprinast and morphine in the spinal cord of rats on the formalin test. Eur J Anaesthesiol. 2006. 23:65–70.
Article15. Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol. 1991. 201:121–122.
Article16. Ortiz MI, Castro-Olguín J, Peña-Samaniego N, Castañeda-Hernández G. Probable activation of the opioid receptor-nitric oxide-cyclic GMP-K+ channels pathway by codeine. Pharmacol Biochem Behav. 2005. 82:695–703.17. Pacheco DF, Reis GM, Francischi JN, Castro MS, Perez AC, Duarte ID. delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta(1) and delta(2) receptors and activation of the larginine/ nitric oxide/cyclic GMP pathway. Life Sci. 2005. 78:54–60.18. Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976. 17:1031–1036.
Article19. Yoon MH, Choi JI. Pharmacologic interaction between cannabinoid and either clonidine or neostigmine in the rat formalin test. Anesthesiology. 2003. 99:701–707.
Article20. Yu SQ, Lundeberg T, Yu LC. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003. 983:13–22.
Article21. Tallarida RJ, Murray RB. Manual of pharmacologic calculations with computer programs. 1987. 2nd ed. New York: Springer-Verlag.22. Yaksh TL. Yaksh TL, Lynch C, Zapol WM, Maze M, Biebuyck JF, Saidman LJ, editors. Preclinical models of nociception. Anesthesia: biologic foundations. 1997. . Philadelphia: Lippincott-Raven;685–718.23. Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990. 11:150–155.
Article24. Ückert S, Hedlund P, Andersson KE, Truss MC, Jonas U, Stief CG. Update on phosphodiesterase (PDE) isoenzymes as pharmacologic targets in urology: present and future. Eur Urol. 2006. 50:1194–1207.
Article25. Mixcoatl-Zecuatl T, Aguirre-Banuelos P, Granados-Soto V. Sildenafil produces antinociception and increases morphine antinociception in the formalin test. Eur J Pharmacol. 2000. 400:81–87.
Article26. Asomoza-Espinosa R, Alonso-Lopez R, Mixcoatl-Zecuatl T, Aguirre-Banuelos P, Torres-Lopez JE, Granados-Soto V. Sildenafil increases diclofenac antinociception in the formalin test. Eur J Pharmacol. 2001. 418:195–200.
Article27. Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil-induced peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Brain Res. 2001. 909:170–178.
Article28. Jain NK, Patil CS, Singh A, Kulkarni SK. Sildenafil, a phosphodiesterase-5 inhibitor, enhances the antinociceptive effect of morphine. Pharmacology. 2003. 67:150–156.
Article29. Patil CS, Singh VP, Kulkarni SK. Modulatory effect of cyclooxygenase inhibitors on sildenafil-induced antinociception. Pharmacology. 2003. 69:183–189.
Article30. Kissin I, Stanski DR, Brown PT, Bradley EL Jr. Pentobarbital-morphine anesthetic interactions in terms of intensity of noxious stimulation required for arousal. Anesthesiology. 1993. 78:744–749.
Article31. Poon A, Sawynok J. Antinociception by adenosine analogs and an adenosine kinase inhibitor: dependence on formalin concentration. Eur J Pharmacol. 1995. 286:177–184.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- GABAB Receptor Modulation on the Antinociception of Intrathecal Sildenafil in the Rat Formalin Test
- Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
- Evaluation for the Effects of Intrathecal Sildenafil on the Formalin- and Thermal-induced Nociception of Rats
- Roles of Adenosine and Serotonin Receptors on the Antinociception of Sildenafil in the Spinal Cord of Rats
- The Role of Opioid Receptor on the Analgesic Action of Intrathecal Sildenafil in Rats