Yonsei Med J.
2011 Jan;52(1):165-172. 10.3349/ymj.2011.52.1.165.
Duration and Magnitude of Extracellular Signal-Regulated Protein Kinase Phosphorylation Determine Adipogenesis or Osteogenesis in Human Bone Marrow-Derived Stem Cells
- Affiliations
-
- 1Department of Orthopaedic Surgery, Yonsei University College of Medicine, Seoul, Korea. ljwos@yuhs.ac
- 2Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1106452
- DOI: http://doi.org/10.3349/ymj.2011.52.1.165
Abstract
- PURPOSE
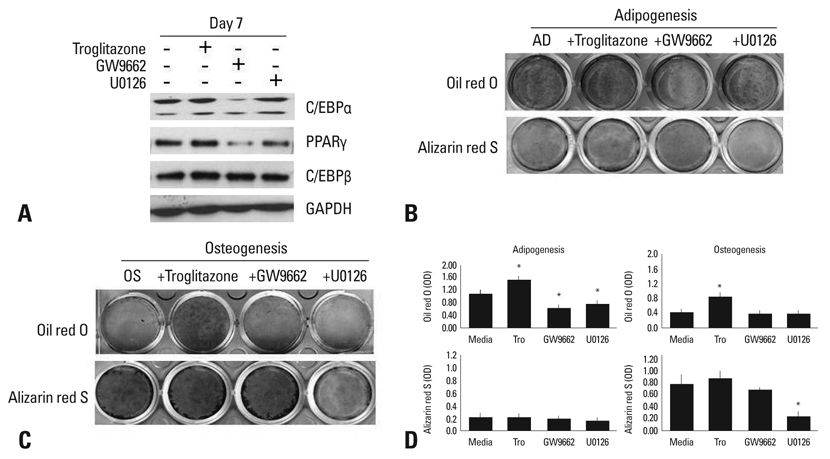
Imbalances between osteogenic and adipogenic differentiation leads to diseases such as osteoporosis. The aim of our study was to demonstrate the differences in extracellular signal-regulated kinase (ERK) phosphorylation during both adipogenesis and osteogenesis of human bone marrow-derived stem cells (BMSCs).
MATERIALS AND METHODS
Using troglitazone, GW9662 and U0126, we investigated their role in hBMSC differentiation to adipogenic and osteogenic fates.
RESULTS
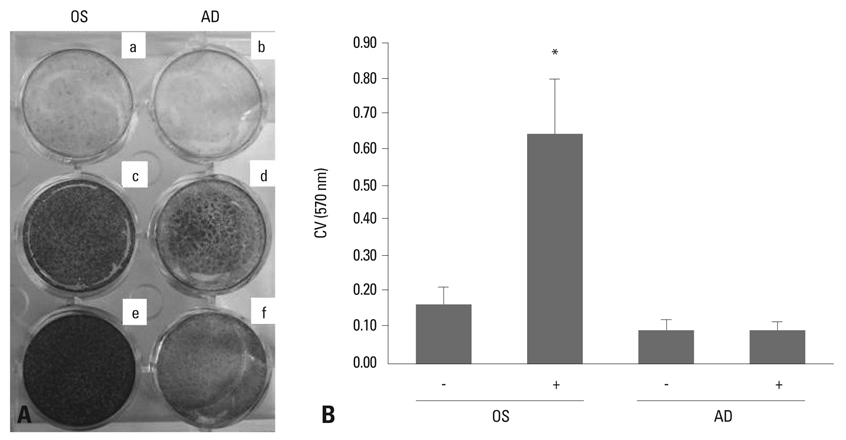
ERK1/2 inhibition by U0126 suppressed proliferator-activated receptor (PPAR)gamma expression and lipid accumulation, while it decreased the mRNA expression of adipogenic genes (lipoprotein lipase, PPARgamma, and adipocyte protein) and osteogenic genes (type I collagen and osteopontin). ERK phosphorylation was transient and decreased during adipogenesis, whereas it occurred steadily during osteogenesis. Troglitazone, a PPARgamma agonist, induced adipogenesis by inhibiting ERK phosphorylation even in an osteogenic medium, suggesting that ERK signaling needs to be shut off in order to proceed with adipose cell commitment. Cell proliferation was greatly increased in osteogenesis but was not changed during adipogenesis, indicating that ERK might play different roles in cellular proliferation and differentiation between the two committed cell types.
CONCLUSION
The duration and magnitude of ERK activation might be a crucial factor for the balance between adipogenesis and osteogenesis in human bone marrow-derived stem cells.
MeSH Terms
-
Adipogenesis/*drug effects/genetics
Adult
Anilides/pharmacology
Bone Marrow Cells/*cytology/drug effects/metabolism
Butadienes/pharmacology
Cell Differentiation/drug effects
Cells, Cultured
Chromans/pharmacology
Extracellular Signal-Regulated MAP Kinases/*metabolism
Female
Humans
Male
Middle Aged
Nitriles/pharmacology
Osteogenesis/*drug effects/genetics
PPAR gamma/agonists/antagonists & inhibitors
Phosphorylation/drug effects
Reverse Transcriptase Polymerase Chain Reaction
Stem Cells/*cytology/drug effects/*metabolism
Thiazolidinediones/pharmacology
Figure
Reference
-
1. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article2. Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000. 21:393–411.
Article3. Chan GK, Deckelbaum RA, Bolivar I, Goltzman D, Karaplis AC. PTHrP inhibits adipocyte differentiation by down-regulating PPAR gamma activity via a MAPK-dependent pathway. Endocrinology. 2001. 142:4900–4909.4. Chin BY, Petrache I, Choi AM, Choi ME. Transforming growth factor beta1 rescues serum deprivation-induced apoptosis via the mitogen-activated protein kinase (MAPK) pathway in macrophages. J Biol Chem. 1999. 274:11362–11368.
Article5. Hauner H, Hochberg Z. Endocrinology of adipose tissue. Horm Metab Res. 2002. 34:605–606.
Article6. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992. 102:341–351.
Article7. Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002. 174:11–20.
Article8. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002. 105:93–98.
Article9. Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J Biol Chem. 2000. 275:9645–9652.
Article10. Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, et al. Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem. 1999. 274:24965–24972.11. Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Binétruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J. 2002. 361:621–627.
Article12. Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2002. 277:46226–46232.13. Kim SH, Choi YR, Park MS, Shin JW, Park KD, Kim SJ, et al. ERK 1/2 activation in enhanced osteogenesis of human mesenchymal stem cells in poly(lactic-glycolic acid) by cyclic hydrostatic pressure. J Biomed Mater Res A. 2007. 80:826–836.
Article14. Hossain MM, Hwang DY, Huang QQ, Sasaki Y, Jin JP. Developmentally regulated expression of calponin isoforms and the effect of h2-calponin on cell proliferation. Am J Physiol Cell Physiol. 2003. 284:C156–C167.
Article15. Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle. 2007. 6:1539–1548.
Article16. Hanlon M, Sturgill TW, Sealy L. ERK2- and p90(Rsk2)-dependent pathways regulate the CCAAT/enhancer-binding protein-beta interaction with serum response factor. J Biol Chem. 2001. 276:38449–38456.
Article17. Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002. 55:693–698.
Article18. Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000. 15:2084–2094.
Article19. Dang ZC, Lowik CW. Differential effects of PD98059 and U0126 on osteogenesis and adipogenesis. J Cell Biochem. 2004. 92:525–533.
Article20. Traverse S, Seedorf K, Paterson H, Marshall CJ, Cohen P, Ullrich A. EGF triggers neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994. 4:694–701.
Article21. Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999. 19:2435–2444.
Article22. Tan PB, Kim SK. Signaling specificity: the RTK/RAS/MAP kinase pathway in metazoans. Trends Genet. 1999. 15:145–149.
Article23. Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995. 80:179–185.
Article24. Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003. 100:44–49.
Article25. Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004. 11:137–153.
Article26. Hipskind RA, Bilbe G. MAP kinase signaling cascades and gene expression in osteoblasts. Front Biosci. 1998. 3:d804–d816.
Article27. Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000. 275:4453–4459.
Article28. Selvamurugan N, Pulumati MR, Tyson DR, Partridge NC. Parathyroid hormone regulation of the rat collagenase-3 promoter by protein kinase A-dependent transactivation of core binding factor alpha1. J Biol Chem. 2000. 275:5037–5042.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transdifferentiation from Adipogenic bone Marrow Derived Stromal Cell to Osteoblast
- Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells
- Sustained Intracellular Acidosis Triggers the Naâº/H⺠Exchager-1 Activation in Glutamate Excitotoxicity
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Various Wavelengths of Light-Emitting Diode Light Regulate the Proliferation of Human Dermal Papilla Cells and Hair Follicles via Wnt/β-Catenin and the Extracellular Signal-Regulated Kinase Pathways