Clin Orthop Surg.
2011 Mar;3(1):48-54. 10.4055/cios.2011.3.1.48.
Long-Term Survivals of Stage IIB Osteosarcoma: A 20-Year Experience in a Single Institution
- Affiliations
-
- 1Department of Orthopedic Surgery, Armed Forces Capital Hospital, Seongnam, Korea.
- 2Department of Orthopaedic Surgery, Kosin University Gospel Hospital, Busan, Korea. jdkim@ns.kosinmed.or.kr
- KMID: 999460
- DOI: http://doi.org/10.4055/cios.2011.3.1.48
Abstract
- BACKGROUND
The purpose of this study is to evaluate the disease-free survival (DFS) and overall survival (OS) of patients with stage IIB osteosarcoma at a single institution for 20 years and to compare the results according to the chemotherapy protocols.
METHODS
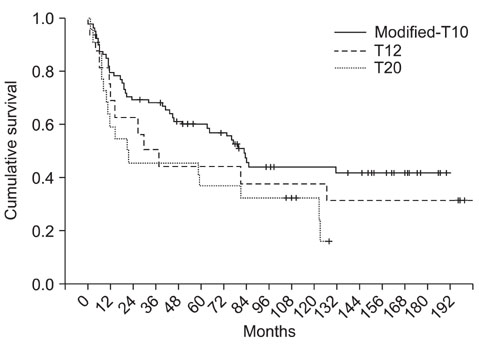
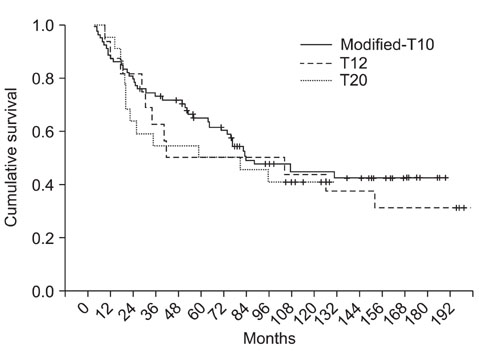
From Jan 1988 to Nov 2008, 167 patients with osteosarcoma were treated at our hospital and among them, 117 patients (67 males and 50 females) with stage IIB osteosarcoma were evaluable. Their mean age was 22.6 years (range, 8 months to 71 years). Seventy-eight cases underwent the modified T10 (M-T10) protocol (group 1), 23 cases underwent the T20 protocol (group 2) and 16 cases underwent the T12 protocol (group 3). The DFS and OS were calculated and compared according to the chemotherapy protocols.
RESULTS
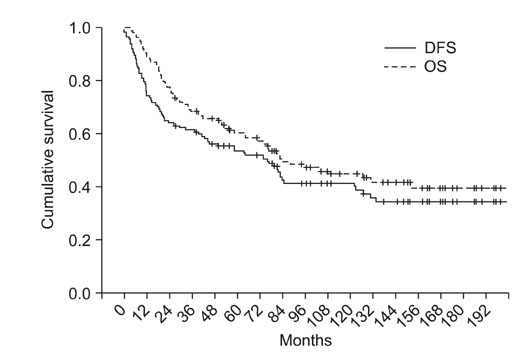
At a mean follow-up of 78.9 months, 63 patients were continuously disease-free (63/117), 6 patients were alive after having metastatic lesions, 7 patients died of other cause and 41 patients died of their disease. The 5- and 10-year OS rates were 60.2% and 44.8%, respectively and the 5- and 10-year DFS rates were 53.5% and 41.4%, respectively. There was no significant difference of the OS and DFS between the chemotherapy protocols (p = 0.692, p = 0.113).
CONCLUSIONS
At present, we achieved success rates close to the internationally accepted DFS and OS. We were able to achieve the higher survival rates using the M-T10 protocol over the 20 years. However, there was no significant difference of results between the chemotherapy protocols. We think the M-T10 protocol will achieve more favorable results in the near future.
MeSH Terms
-
Adolescent
Adult
Aged
Antineoplastic Combined Chemotherapy Protocols/administration & dosage/*therapeutic use
Bleomycin/administration & dosage
Bone Neoplasms/*drug therapy/*mortality/surgery
Chemotherapy, Adjuvant
Child
Child, Preschool
Cyclophosphamide/administration & dosage
Dactinomycin/administration & dosage
Disease-Free Survival
Doxorubicin/administration & dosage
Female
Follow-Up Studies
Humans
Infant
Kaplan-Meier Estimate
Leucovorin/administration & dosage
Male
Methotrexate/administration & dosage
Middle Aged
Neoadjuvant Therapy
Osteosarcoma/*drug therapy/*mortality/surgery
Survival Rate
Vincristine/administration & dosage
Young Adult
Figure
Reference
-
1. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005. 41(18):2836–2845.
Article2. Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986. 314(25):1600–1606.
Article3. Rosen G, Caparros B, Huvos AG, et al. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982. 49(6):1221–1230.
Article4. Winkler K, Beron G, Kotz R, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: results of a Cooperative German/Austrian study. J Clin Oncol. 1984. 2(6):617–624.
Article5. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000. 18(24):4016–4027.
Article6. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002. 20(3):776–790.
Article7. Petrilli AS, de Camargo B, Filho VO, et al. Results of the Brazilian Osteosarcoma Treatment Group Studies III and IV: prognostic factors and impact on survival. J Clin Oncol. 2006. 24(7):1161–1168.
Article8. Meyers PA, Gorlick R, Heller G, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998. 16(7):2452–2458.
Article9. Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980. (153):106–120.
Article10. Rosen G. Neoadjuvant chemotherapy for osteogenic sarcoma: a model for the treatment of other highly malignant neoplasms. Recent Results Cancer Res. 1986. 103:148–157.
Article11. Rosen G, Marcove RC, Huvos AG, et al. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983. 106:Suppl. 55–67.
Article12. Rosen G, Huvos AG, Mosende C, et al. Chemotherapy and thoractomy for metastatic osteogenic sarcoma: a model for adjuvant chemotherapy and the rationale for the timing of thoracic surgery. Cancer. 1978. 41(3):841–849.
Article13. Rosen G. Preoperative (neoadjuvant) chemotherapy for osteogenic sarcoma: a ten year experience. Orthopedics. 1985. 8(5):659–664.
Article14. Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006. 32(6):423–436.
Article15. Fuchs B, Hoekzema N, Larson DR, Inwards CY, Sim FH. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009. 467(2):510–518.
Article16. Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. 2006. 6(7):1075–1085.
Article17. Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976. 37(1):1–11.
Article18. Campanacci M, Bacci G, Bertoni F, Picci P, Minutillo A, Franceschi C. The treatment of osteosarcoma of the extremities: twenty year's experience at the Istituto Ortopedico Rizzoli. Cancer. 1981. 48(7):1569–1581.
Article19. Avella M, Bacci G, McDonald DJ, Di Scioscio M, Picci P, Campanacci M. Adjuvant chemotherapy with six drugs (adriamycin, methotrexate, cisplatinum, bleomycin, cyclophosphamide and dactinomycin) for non-metastatic high grade osteosarcoma of the extremities: results of 32 patients and comparison to 127 patients concomitantly treated with the same drugs in a neoadjuvant form. Chemioterapia. 1988. 7(2):133–137.20. Meyers PA, Heller G, Healey J, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992. 10(1):5–15.
Article21. Bacci G, Ferrari S, Longhi A, et al. High dose ifosfamide in combination with high dose methotrexate, adriamycin and cisplatin in the neoadjuvant treatment of extremity osteosarcoma: preliminary results of an Italian Sarcoma Group/Scandinavian Sarcoma Group pilot study. J Chemother. 2002. 14(2):198–206.
Article22. Smeele LE, Kostense PJ, van der Waal I, Snow GB. Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol. 1997. 15(1):363–367.
Article23. Fuchs N, Bielack SS, Epler D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998. 9(8):893–899.
Article24. Smeland S, Wiebe T, Bohling T, Brosjo O, Jonsson K, Alvegard TA. Chemotherapy in osteosarcoma: the Scandinavian Sarcoma Group experience. Acta Orthop Scand Suppl. 2004. 75(311):92–98.25. Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol. 2005. 23(9):2004–2011.
Article26. de Kraker J, Voute PA. Experience with ifosfamide in paediatric tumours. Cancer Chemother Pharmacol. 1989. 24:Suppl 1. S28–S29.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Survival of Stage IIB Osteosarcoma-Limb-Salvage vs Ampuration
- Distal Radius Osteosarcoma
- Osteosarcoma, survivorship following stage and chemotherapeutic regimen: 13 year experience of Korea Cancer Center Hospital
- Pathological responses to preoperative high-dose methotrexate chemotherapy in osteosarcoma: experience in Korea cancer hospital
- Repeated Pulmonary Metastasectomy in Patients with Osteosarcoma