Yonsei Med J.
2007 Jun;48(3):396-404. 10.3349/ymj.2007.48.3.396.
Vaccination against Murine Toxoplasmosis Using Recombinant Toxoplasma gondii SAG3 Antigen Alone or in Combination with Quil A
- Affiliations
-
- 1Department of Infection Biology, College of Medicine, Chungnam National University, Daejeon, Korea. yhalee@cnu.ac.kr
- 2Department of Pediatrics, College of Medicine, Chungnam National University, Daejeon, Korea.
- 3Department of Parasitology, Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Department of Parasitology, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 724093
- DOI: http://doi.org/10.3349/ymj.2007.48.3.396
Abstract
- PURPOSE
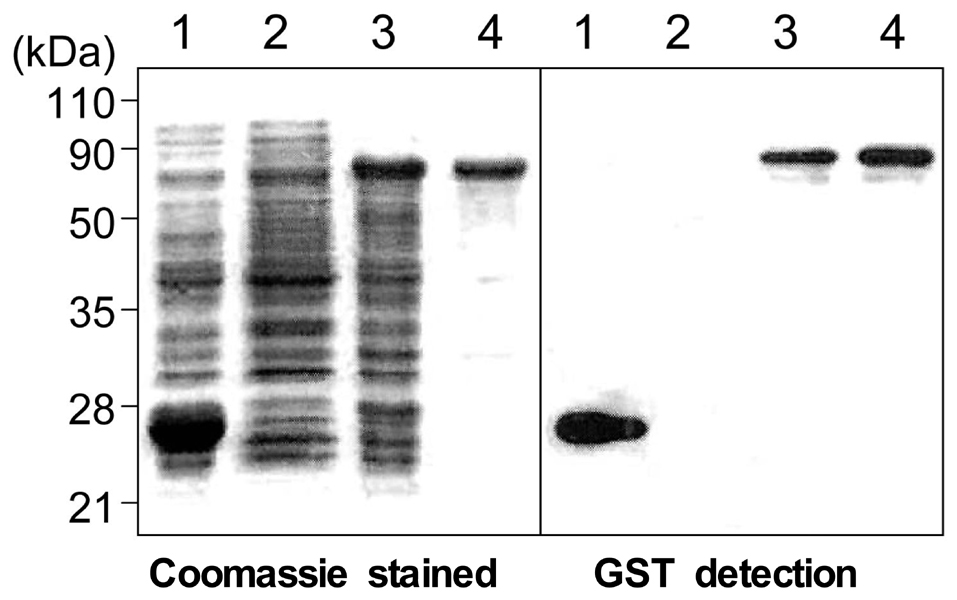
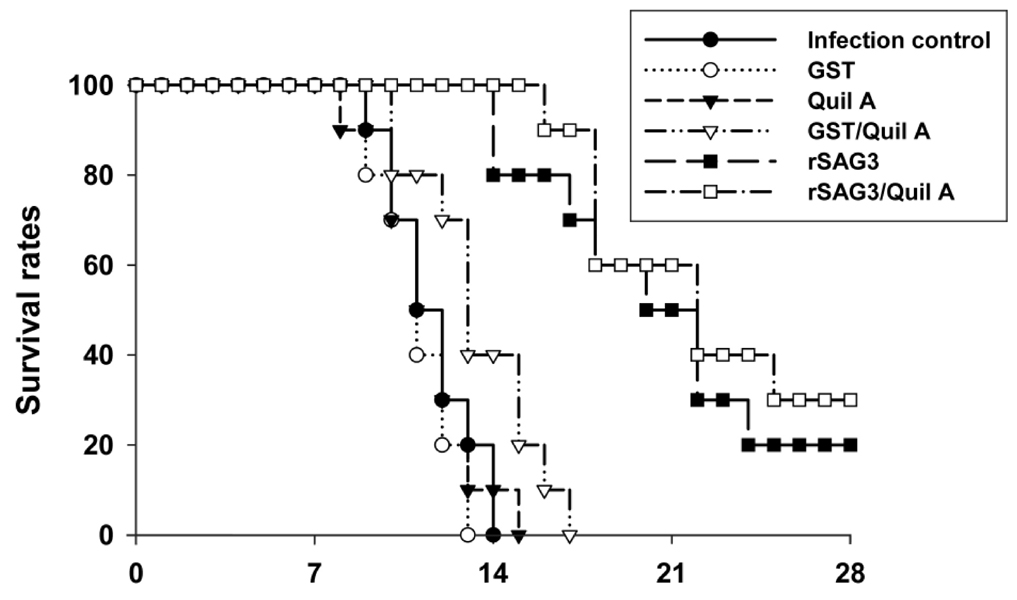
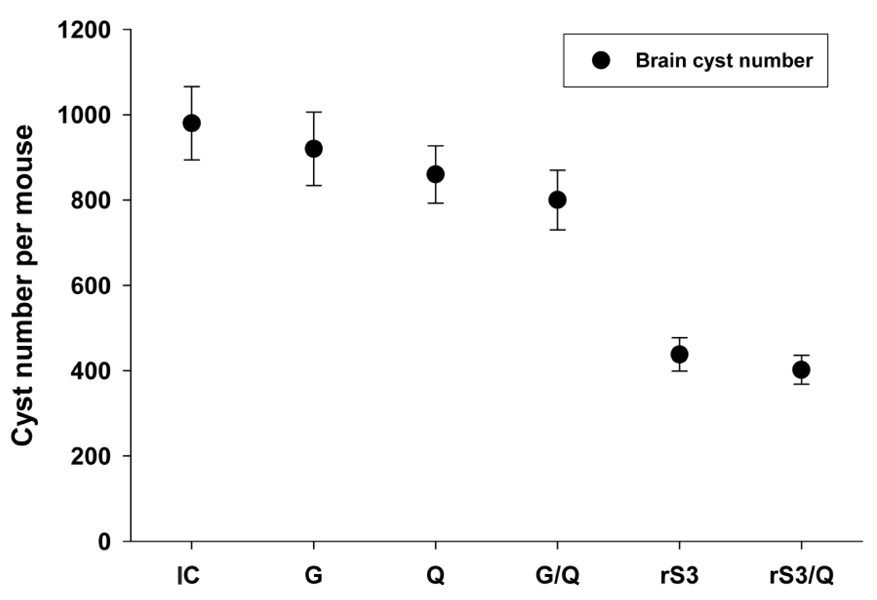
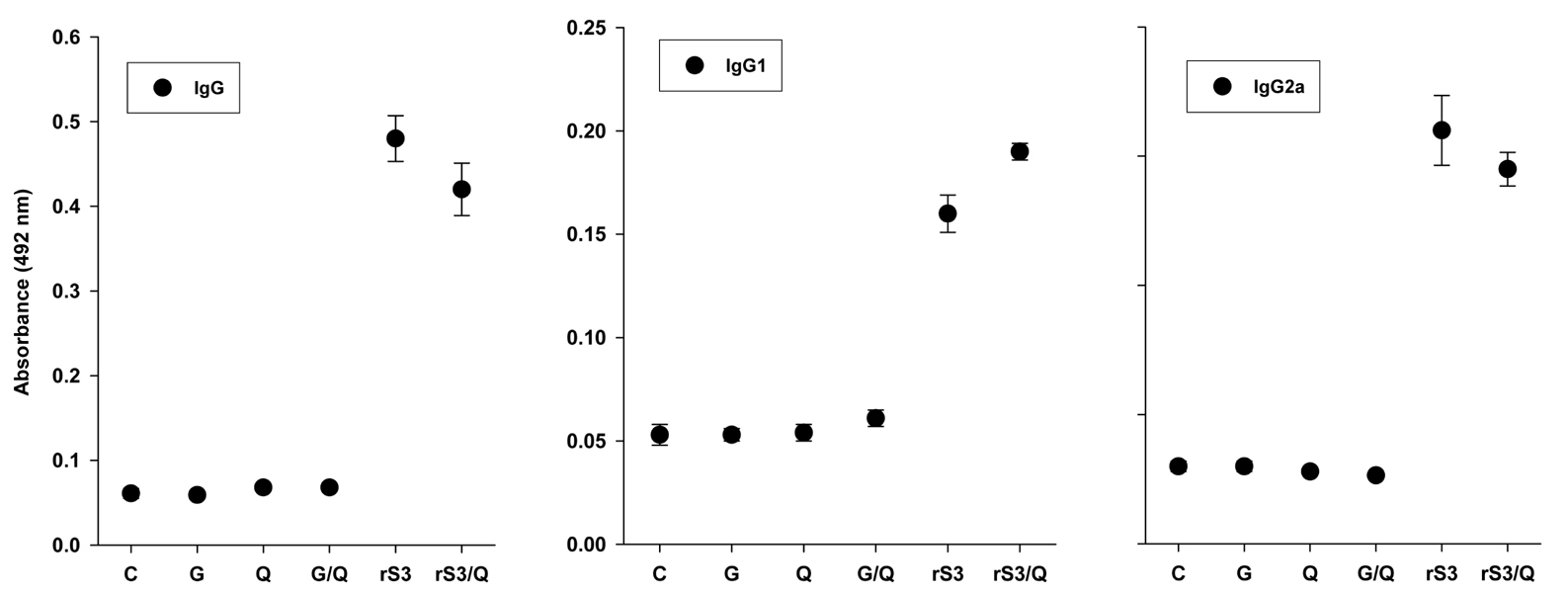
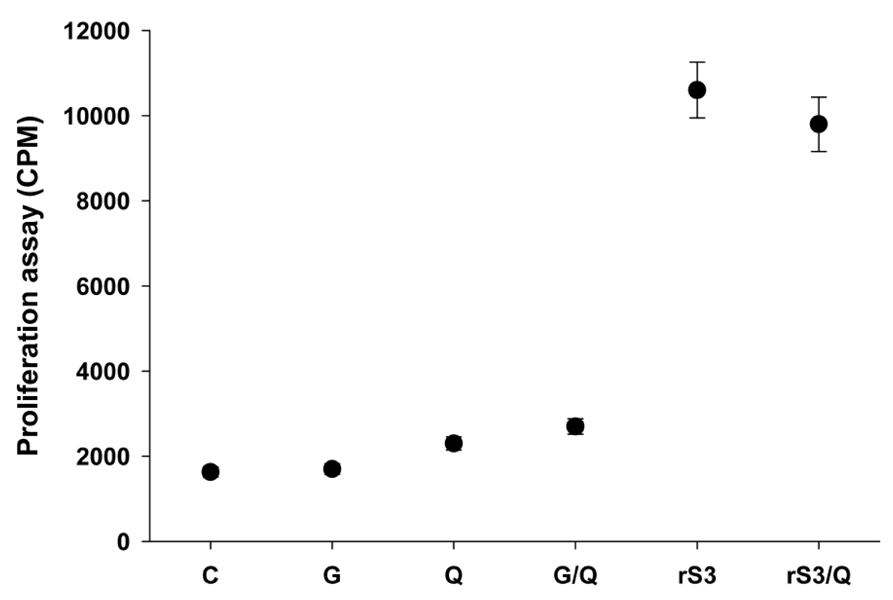
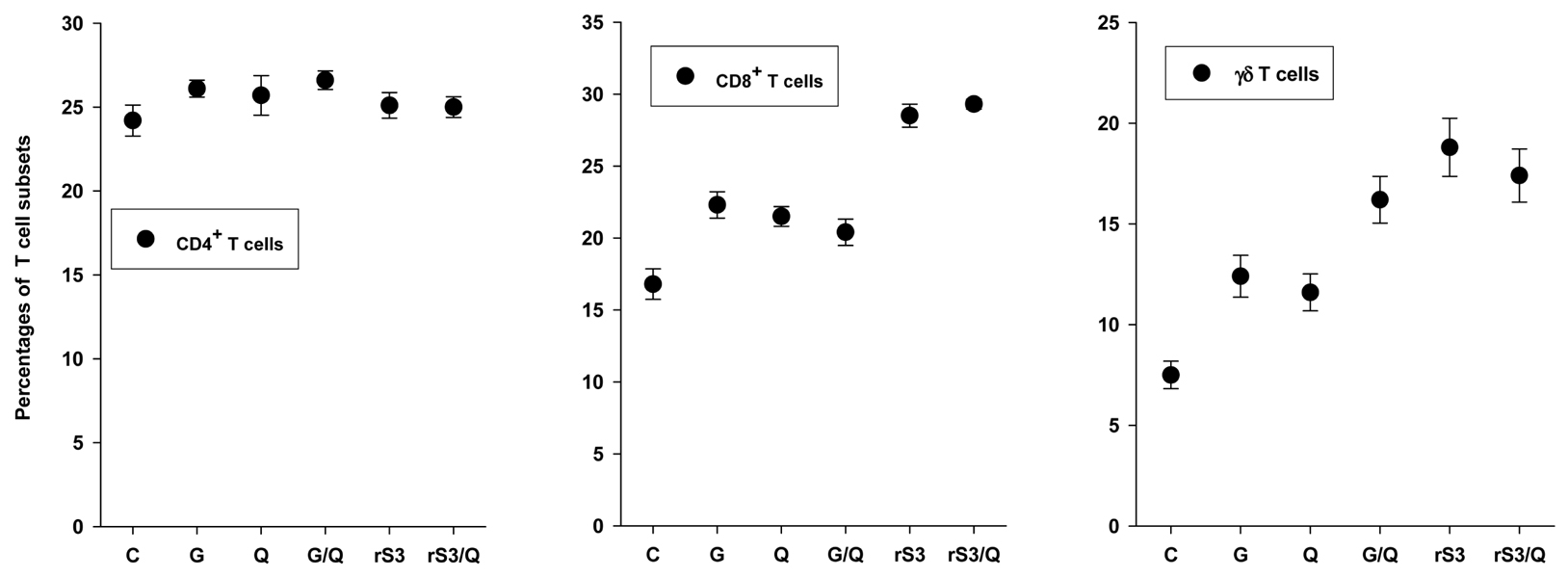
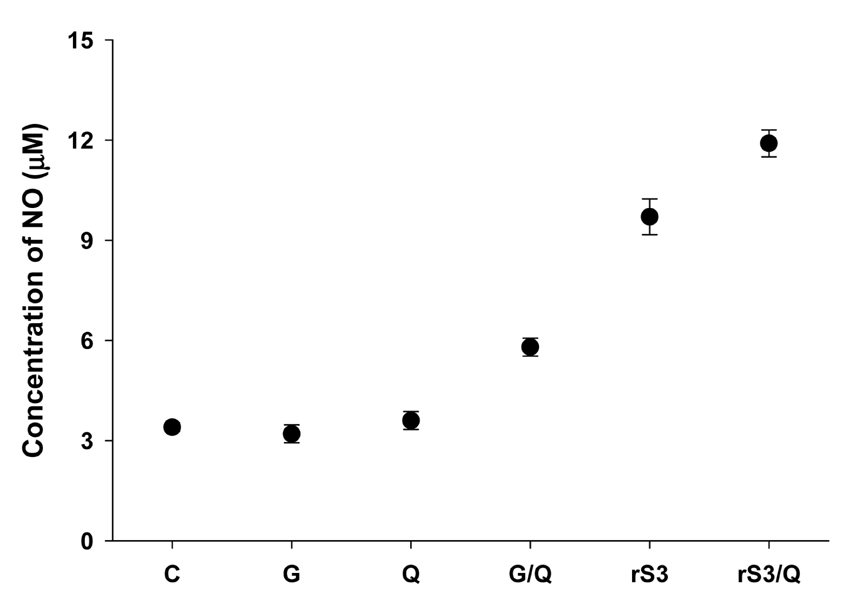
Surface antigen 3 (SAG3) of Toxoplasma gondii is very similar in structure to the major surface antigen 1 (SAG1). Although numerous studies have supported the importance of SAG1 in protection against T. gondii infection, few reports exist on SAG3. MATERIALS AND METHODS: Glutathione-S-transferase (GST)-fused SAG3 of T. gondii (rSAG3) were immunized into BALB/c mice alone or in combination with Quil A (rSAG3/Quil A), and then evaluated the protective immunity in vivo and in vitro against murine toxoplasmosis. RESULTS: Immunization with rSAG3 or rSAG3/Quil A resulted in significantly more survival days and fewer brain cysts after challenge with T. gondii compared to an infected control group. Mice immunized with rSAG3 alone or in combination with Quil A produced significantly more specific IgG2a antibody, whereas specific IgG1 antibody titers did not increase. The percentage of CD8+ T cells, IFN-gamma mRNA expression, and nitric oxide production significantly increased in rSAG3- and rSAG3/Quil A-immunized mice. CONCLUSION: These results indicate that vaccination with Toxoplasma rSAG3 results in partial protective immunity against T. gondii infection through induction of a Th1-type immune response, and that protective immunity is accelerated by the modulating effects of Quil A.
MeSH Terms
-
Animals
Antigens, Protozoan/genetics/*immunology/metabolism
Bacterial Proteins/genetics/immunology/metabolism
Blotting, Western
Enzyme-Linked Immunosorbent Assay
Female
Flow Cytometry
Immunoglobulin G/immunology
Interferon-gamma/metabolism
Mice
Mice, Inbred BALB C
Nitric Oxide/metabolism
Protozoan Proteins/genetics/immunology/metabolism
Recombinant Fusion Proteins/genetics/immunology/metabolism
Reverse Transcriptase Polymerase Chain Reaction
Saponins/*immunology
Toxoplasma/growth & development/*immunology
Toxoplasmosis, Animal/*immunology/metabolism/microbiology
Vaccination/*methods
Figure
Reference
-
1. Filisetti D, Candolfi E. Immune response to Toxoplasma gondii. Ann 1st Super Sanita. 2004. 40:71–80.2. Lekutis C, Ferguson DJ, Grigg ME, Camps M, Boothroyd JC. Surface antigens of Toxoplasma gondii: variations on a theme. Int J Parasitol. 2001. 31:1285–1292.3. Kasper LH, Crabb JH, Pfefferkorn ER. Purification of a major membrane protein of Toxoplasma gondii by immunoabsorption with a monoclonal antibody. J Immunol. 1983. 130:2407–2412.4. Parmley SF, Sgarlato GD, Mark J, Prince JB, Remington JS. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol. 1992. 3:1127–1133.5. Cesbron-Delauw MF, Tomavo S, Beauchamps P, Fourmaux MP, Camus D, Capron A, et al. Similarities between the primary structures of two distinct major surface proteins of Toxoplasma gondii. J Biol Chem. 1994. 269:16217–16222.
Article6. Letscher-Bru V, Villard O, Risse B, Zauke M, Klein JP, Kien TT. Protective effect of vaccination with a combination of recombinant surface antigen 1 and interleukin-12 against toxoplasmosis in mice. Infect Immun. 1998. 66:4503–4506.
Article7. Couper KN, Nielsen HV, Petersen E, Roberts F, Roberts CW, Alexander J. DNA vaccination with the immunodominant tachyzoite surface antigen (SAG-1) protects against adult acquired Toxoplasma gondii infection but does not prevent maternofoetal transmission. Vaccine. 2003. 21:2813–2820.
Article8. Mohamed RM, Aosai F, Chen M, Mun HS, Norose K, Belal US, et al. Induction of protective immunity by DNA vaccination with Toxoplasma gondii HSP70, HSP30 and SAG1 genes. Vaccine. 2003. 21:2852–2861.
Article9. Mevelec MN, Bout D, Desolme B, Marchand H, Magne R, Bruneel O, et al. Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine. 2005. 23:4489–4499.
Article10. Kasper LH, Currie KM, Bradley MS. An unexpected response to vaccination with a purified major membrane tachyzoite antigen (P30) of Toxoplasma gondii. J Immunol. 1985. 134:3426–3431.11. Jacquet A, Coulon L, De Neve J, Daminet V, Haumont M, Garcia L, et al. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol. 2001. 116:35–44.
Article12. Dzierszinski F, Mortuaire M, Cesbron-Delauw MF, Tomavo S. Targeted disruption of the glycosylphosphatidylinositol-anchored surface antigen SAG3 gene in Toxoplasma gondii decreases host cell adhesion and drastically reduces virulence in mice. Mol Microbiol. 2000. 37:574–582.
Article13. Caetano BC, Bruna-Romero O, Fux B, Mendes EA, Penido ML, Gazzinelli RT. Vaccination with replication-deficient recombinant adenoviruses encoding the main surface antigens of Toxoplasma gondii induces immune response and protection against infection in mice. Hum Gene Ther. 2006. 17:415–426.
Article14. Deckert-Schluter M, Albrecht S, Hof H, Wiestler OD, Schluter D. Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenic strains of mice. Immunology. 1995. 85:408–418.15. Hayashi S, Chan CC, Gazzinelli R, Roberge FG. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996. 156:1476–1481.16. Burns JM Jr, Dunn PD, Russo DM. Protective immunity against Plasmodium yoelii malaria induced by immunization with particulate blood-stage antigens. Infect Immun. 1997. 65:3138–3145.
Article17. Behboudi S, Morein B, Villacres-Eriksson MC. Quillaja saponin formulations that stimulate proinflammatory cytokines elicit a potent acquired cell-mediated immunity. Scand J Immunol. 1999. 50:371–377.
Article18. Morein B, Bengtsson KL. Immunomodulation by iscoms, immune stimulating complexes. Methods. 1999. 19:94–102.
Article19. Nguyen TD, Bigaignon G, Van Broeck J, Vercammen M, Nguyen TN, Delmee M, et al. Acute and chronic phases of Toxoplasma gondii infection in mice modulate the host immune responses. Infect Immun. 1998. 66:2991–2995.
Article20. Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005. 5:162–170.
Article21. Wang X, Claflin J, Kang H, Suzuki Y. Importance of CD8(+)Vβ8(+) T cells in IFNγ-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J Interferon Cytokine Res. 2005. 25:338–344.
Article22. Abou-Bacar A, Pfaff AW, Letscher-Bru V, Filisetti D, Rajapakse R, Antoni E, et al. Role of gamma interferon and T cells in congenital Toxoplasma transmission. Parasite Immunol. 2004. 26:315–318.
Article23. Denkers EY. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect. 1999. 1:699–708.
Article24. Casciotti L, Ely KH, Williams ME, Khan IA. CD8+-T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect Immun. 2002. 70:434–443.
Article25. Rodrigues MM, Boscardin SB, Vasconcelos JR, Hiyane MI, Salay G, Soares IS. Importance of CD8 T cell-mediated immune response during intracellular parasitic infections and its implications for the development of effective vaccines. An Acad Bras Cienc. 2003. 75:443–468.
Article26. Luder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int J Parasitol. 2003. 33:833–844.27. Dumont AR, Kalfayan LH, Sekaly RP. Modulation of immune responses-strategies for optimising vaccines. Expert Opin Biol Ther. 2004. 4:627–630.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Recombinant SAG1, SAG2, and SAG3 Antigens for Serodiagnosis of Toxoplasmosis
- Immunological properties of the 30 kDa antigen of toxoplasma gondii
- Inhibitory Effects of Toxoplasma Antigen on Proliferation and Invasion of Human Glioma Cells
- A role of carboxy-terminal region of Toxoplasma gondii-heat shock protein 70 in enhancement of T. gondii infection in mice
- Production and Evaluation of Toxoplasma gondii Recombinant GRA7 for Serodiagnosis of Human Infections