Endocrinol Metab.
2024 Jun;39(3):511-520. 10.3803/EnM.2023.1915.
Lipid Variability Induces Endothelial Dysfunction by Increasing Inflammation and Oxidative Stress
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Medical Informatics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2556643
- DOI: http://doi.org/10.3803/EnM.2023.1915
Abstract
- Background
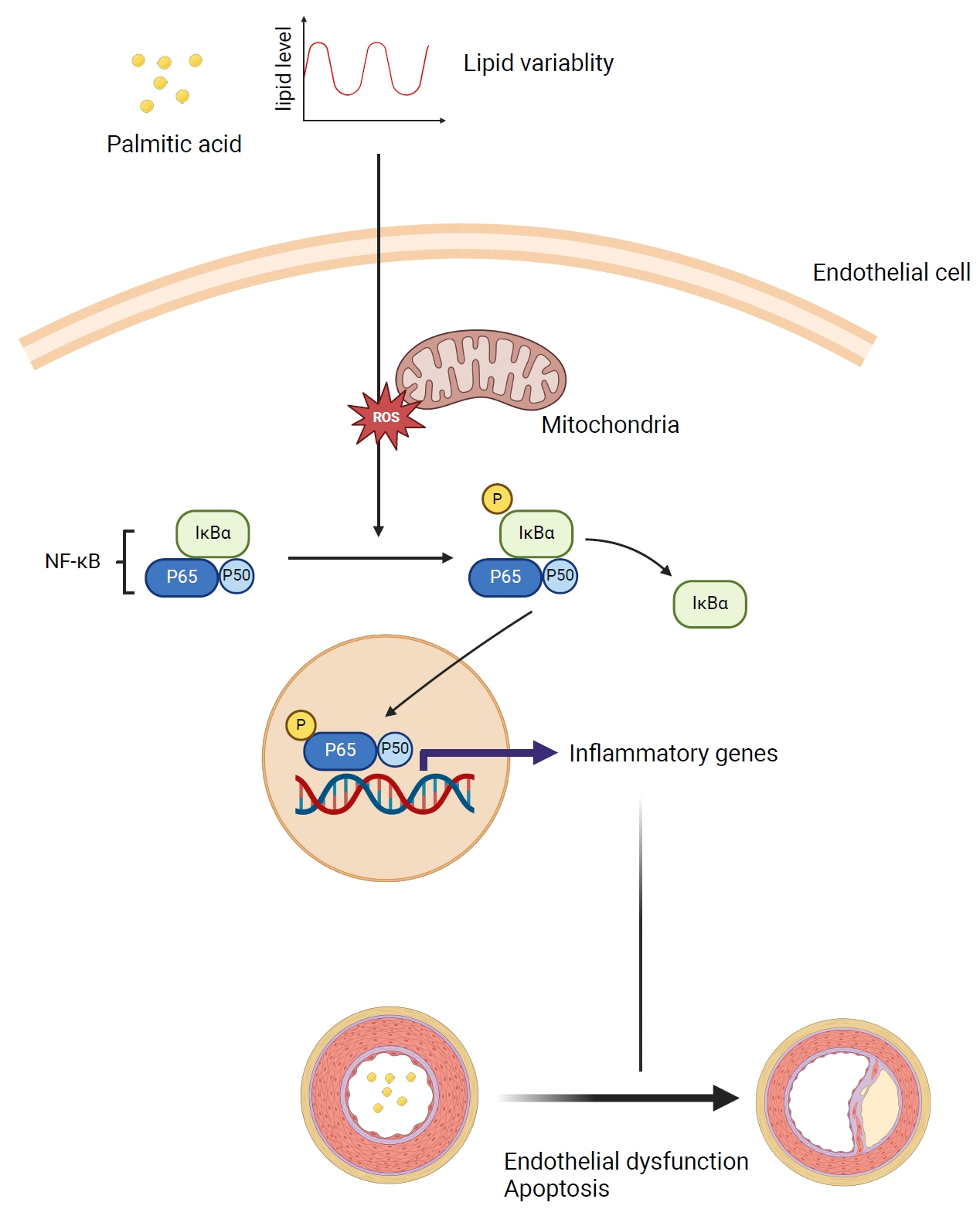
This study investigates the impact of fluctuating lipid levels on endothelial dysfunction.
Methods
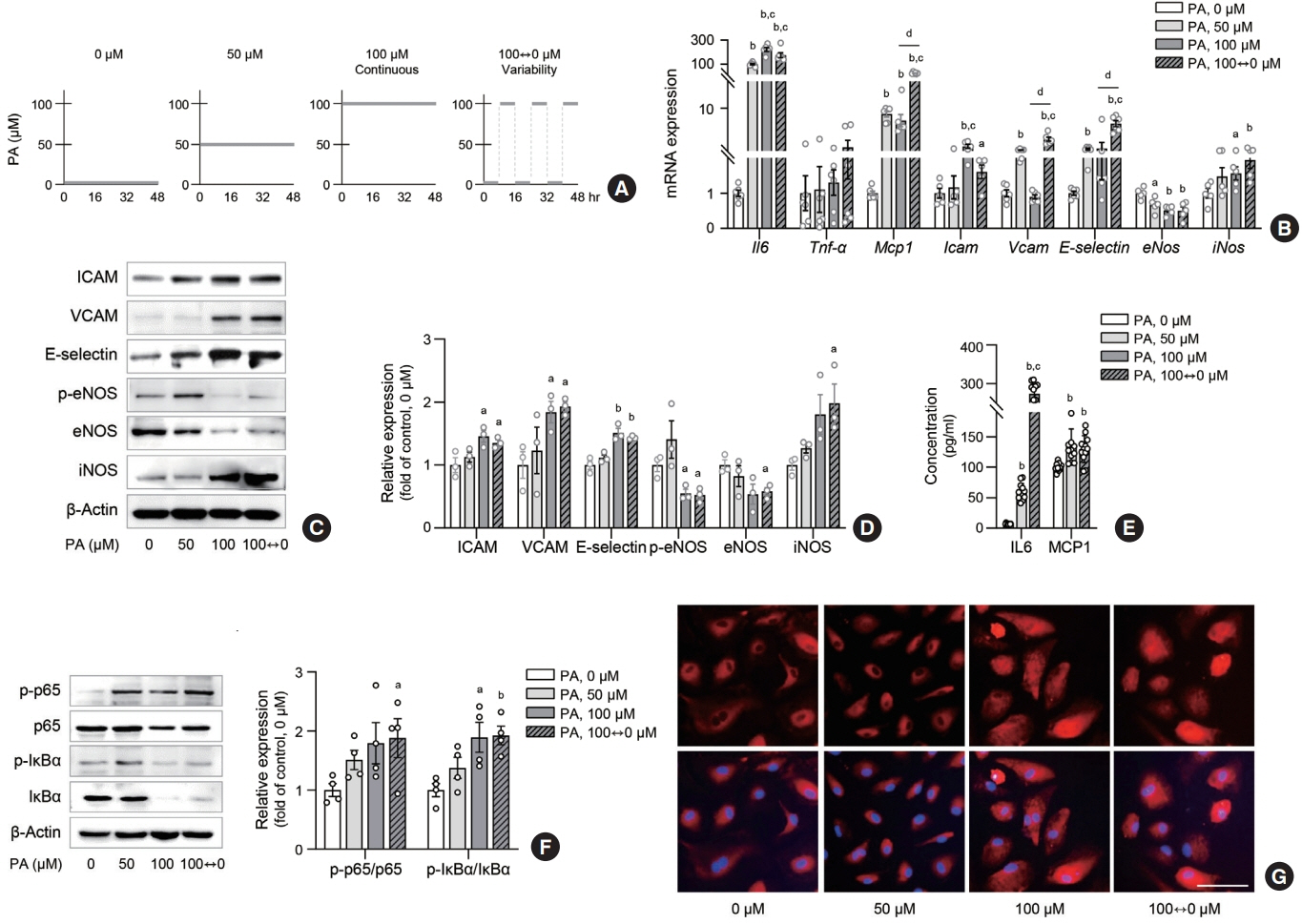
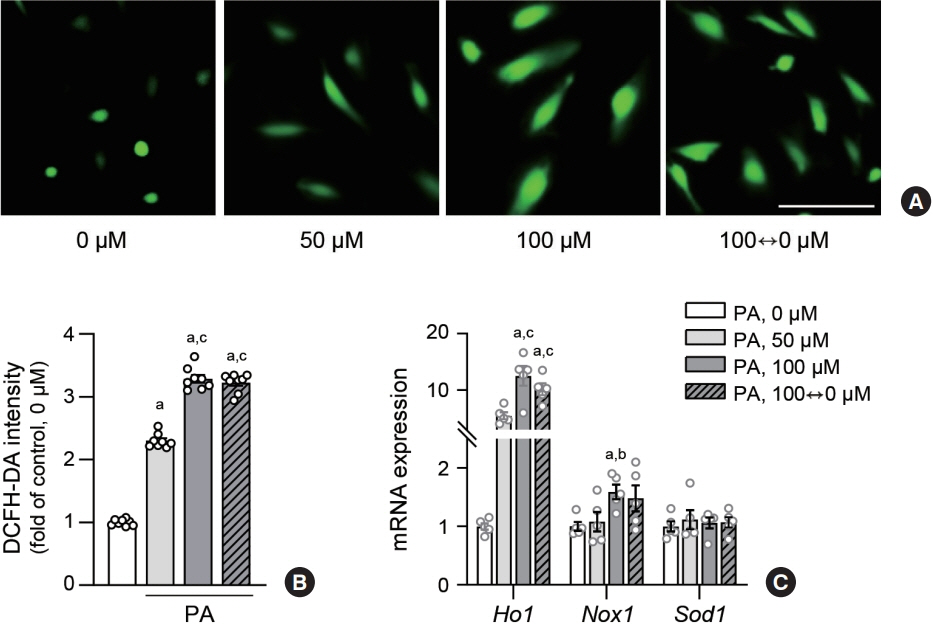
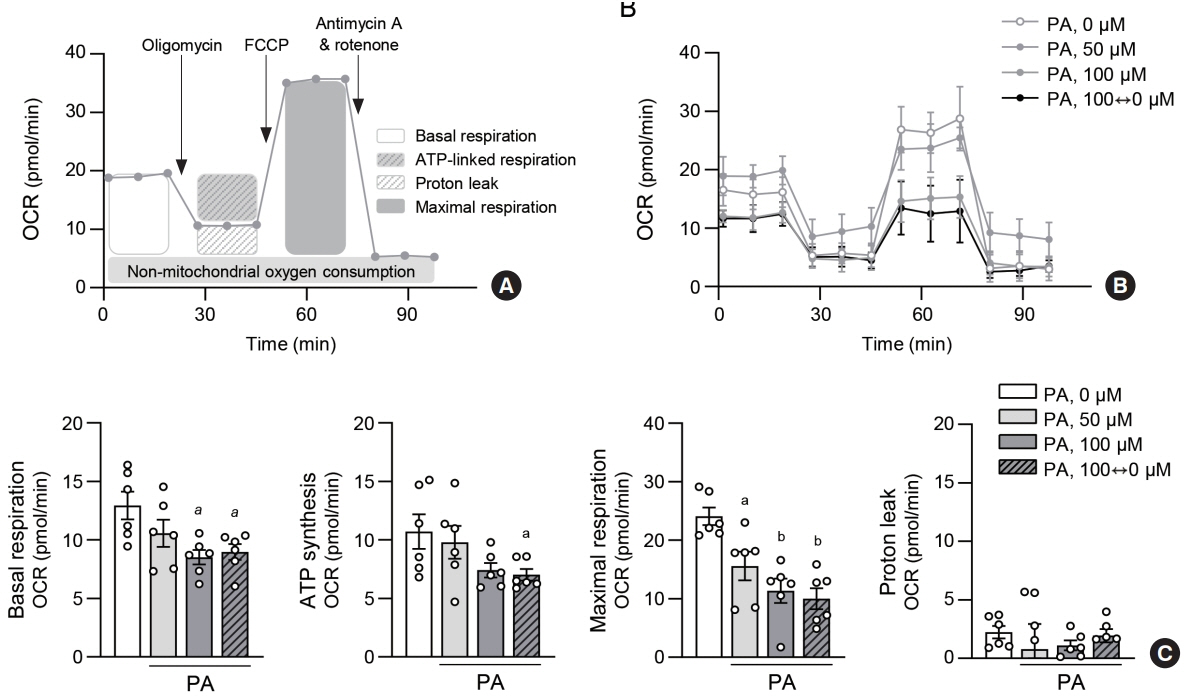
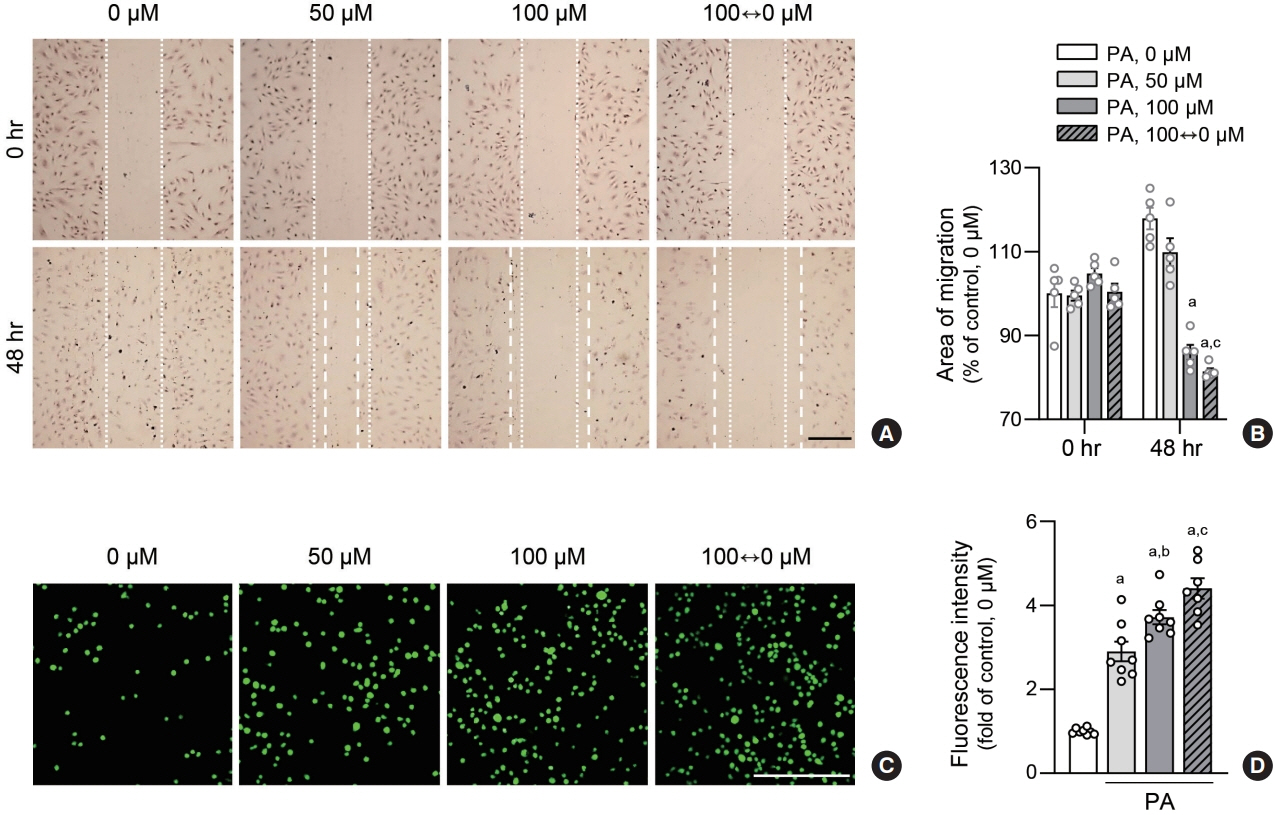
Human aortic and umbilical vein endothelial cells were cultured under varying palmitic acid (PA) concentrations: 0, 50, and 100 μM, and in a variability group alternating between 0 and 100 μM PA every 8 hours for 48 hours. In the lipid variability group, cells were exposed to 100 μM PA during the final 8 hours before analysis. We assessed inflammation using real-time polymerase chain reaction, Western blot, and cytokine enzyme-linked immunosorbent assay (ELISA); reactive oxygen species (ROS) levels with dichlorofluorescin diacetate assay; mitochondrial function through oxygen consumption rates via XF24 flux analyzer; and endothelial cell functionality via wound healing and cell adhesion assays. Cell viability was evaluated using the MTT assay.
Results
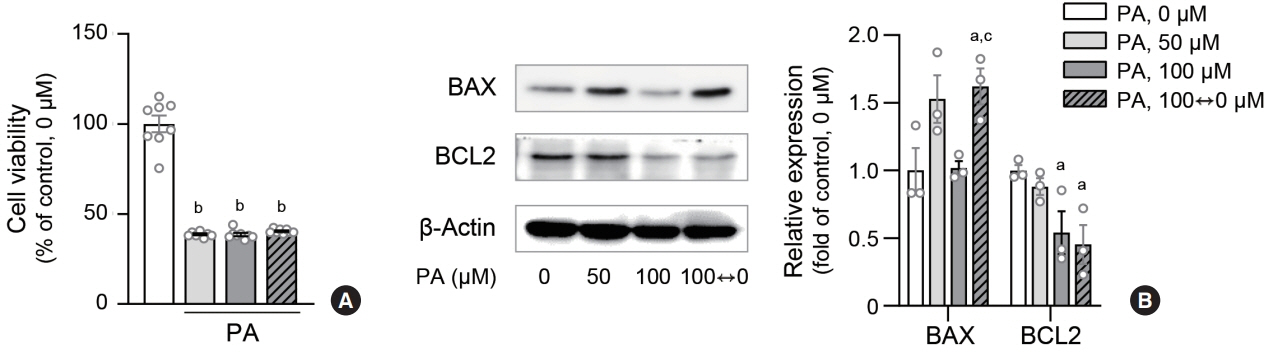
Variable PA levels significantly upregulated inflammatory genes and adhesion molecules (Il6, Mcp1, Icam, Vcam, E-selectin, iNos) at both transcriptomic and protein levels in human endothelial cells. Oscillating lipid levels reduced basal respiration, adenosine triphosphate synthesis, and maximal respiration, indicating mitochondrial dysfunction. This lipid variability also elevated ROS levels, contributing to a chronic inflammatory state. Functionally, these changes impaired cell migration and increased monocyte adhesion, and induced endothelial apoptosis, evidenced by reduced cell viability, increased BAX, and decreased BCL2 expression.
Conclusion
Lipid variability induce endothelial dysfunction by elevating inflammation and oxidative stress, providing mechanistic insights into how lipid variability increases cardiovascular risk.
Keyword
Figure
Cited by 1 articles
-
Lipid Swings Provoke Vascular Inflammation
Jae-Han Jeon
Endocrinol Metab. 2024;39(3):448-449. doi: 10.3803/EnM.2024.302.
Reference
-
1. Eelen G, de Zeeuw P, Treps L, Harjes U, Wong BW, Carmeliet P. Endothelial cell metabolism. Physiol Rev. 2018; 98:3–58.
Article2. Ghosh A, Gao L, Thakur A, Siu PM, Lai CW. Role of free fatty acids in endothelial dysfunction. J Biomed Sci. 2017; 24:50.
Article3. Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007; 7:803–15.
Article4. Gimbrone MA Jr, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013; 22:9–15.
Article5. Mortensen MB, Dzaye O, Botker HE, Jensen JM, Maeng M, Bentzon JF, et al. Low-density lipoprotein cholesterol is predominantly associated with atherosclerotic cardiovascular disease events in patients with evidence of coronary atherosclerosis: the Western Denmark Heart Registry. Circulation. 2023; 147:1053–63.
Article6. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease: six year follow-up experience. The Framingham Study. Ann Intern Med. 1961; 55:33–50.
Article7. Prospective Studies Collaboration, Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007; 370:1829–39.
Article8. Zhang Y, Pletcher MJ, Vittinghoff E, Clemons AM, Jacobs DR Jr, Allen NB, et al. Association between cumulative low-density lipoprotein cholesterol exposure during young adulthood and middle age and risk of cardiovascular events. JAMA Cardiol. 2021; 6:1406–13.
Article9. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res. 2016; 118:547–63.10. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014; 384:626–35.
Article11. Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996; 3:213–9.
Article12. Chen X, Liu L, Palacios G, Gao J, Zhang N, Li G, et al. Plasma metabolomics reveals biomarkers of the atherosclerosis. J Sep Sci. 2010; 33:2776–83.
Article13. Feinleib M. Seven countries: a multivariate analysis of death and coronary heart disease. JAMA. 1981; 245:511–2.
Article14. Kim MK, Han K, Kim HS, Park YM, Kwon HS, Yoon KH, et al. Cholesterol variability and the risk of mortality, myocardial infarction, and stroke: a nationwide population-based study. Eur Heart J. 2017; 38:3560–6.
Article15. Bangalore S, Breazna A, DeMicco DA, Wun CC, Messerli FH; TNT Steering Committee and Investigators. Visit-tovisit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol. 2015; 65:1539–48.16. Boey E, Gay GM, Poh KK, Yeo TC, Tan HC, Lee CH. Visitto-visit variability in LDL- and HDL-cholesterol is associated with adverse events after ST-segment elevation myocardial infarction: a 5-year follow-up study. Atherosclerosis. 2016; 244:86–92.
Article17. Rhee M, Kim JW, Lee MW, Yoon KH, Lee SH. Preadipocyte factor 1 regulates adipose tissue browning via TNF-αconverting enzyme-mediated cleavage. Metabolism. 2019; 101:153977.
Article18. Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017; 2:17023.
Article19. Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–81.
Article20. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017; 376:1713–22.
Article21. Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999; 341:410–8.
Article22. Amini M, Zayeri F, Salehi M. Trend analysis of cardiovascular disease mortality, incidence, and mortality-to-incidence ratio: results from global burden of disease study 2017. BMC Public Health. 2021; 21:401.
Article23. Kim K, Ginsberg HN, Choi SH. New, novel lipid-lowering agents for reducing cardiovascular risk: beyond statins. Diabetes Metab J. 2022; 46:517–32.
Article24. Park J, Kang M, Ahn J, Kim MY, Choi MS, Lee YB, et al. Mean and variability of lipid measurements and risk for development of subclinical left ventricular diastolic dysfunction. Diabetes Metab J. 2022; 46:286–96.
Article25. Gimbrone MA Jr, Garcia-Cardena G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016; 118:620–36.
Article26. Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA Jr. Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987; 84:9238–42.
Article27. Gahmberg CG, Tolvanen M, Kotovuori P. Leukocyte adhesion: structure and function of human leukocyte beta2-integrins and their cellular ligands. Eur J Biochem. 1997; 245:215–32.28. Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001; 107:1209–10.
Article29. Bischoff J. Cell adhesion and angiogenesis. J Clin Invest. 1997; 99:373–6.
Article30. Stromblad S, Cheresh DA. Cell adhesion and angiogenesis. Trends Cell Biol. 1996; 6:462–8.
Article31. Maus U, Henning S, Wenschuh H, Mayer K, Seeger W, Lohmeyer J. Role of endothelial MCP-1 in monocyte adhesion to inflamed human endothelium under physiological flow. Am J Physiol Heart Circ Physiol. 2002; 283:H2584–91.
Article32. Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003; 42:1075–81.33. Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018; 100:1–19.
Article34. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000; 87:840–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Endothelial Dysfunction in the Pathophysiology and Cerebrovascular Effects of Migraine: A Narrative Review
- Endothelial cell autophagy in the context of disease development
- Clinical Implications of Glucose Variability: Chronic Complications of Diabetes
- Unbalanced Redox With Autophagy in Cardiovascular Disease
- The Role of Oxidative Stress in the Pathogenesis of Asthma