Korean J Physiol Pharmacol.
2024 May;28(3):229-237. 10.4196/kjpp.2024.28.3.229.
Pectolinarigenin ameliorated airway inflammation and airway remodeling to exhibit antitussive effect
- Affiliations
-
- 1Department of Respiratory and Critical Care Medicine, Zhenjiang Hospital of Integrated Traditional Chinese and Western Medicine, Zhenjiang, Jiangsu 212000, China
- 2Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital, Nanjing Medical University, Nanjing, Jiangsu 210029, China
- 3Department of Neurology, Zhenjiang Hospital of Integrated Traditional Chinese and Western Medicine, Zhenjiang, Jiangsu 212000, China
- 4Department of Clinical Laboratory, Zhenjiang Hospital of Integrated Traditional Chinese and Western Medicine, Zhenjiang, Jiangsu 212000, China
- KMID: 2555665
- DOI: http://doi.org/10.4196/kjpp.2024.28.3.229
Abstract
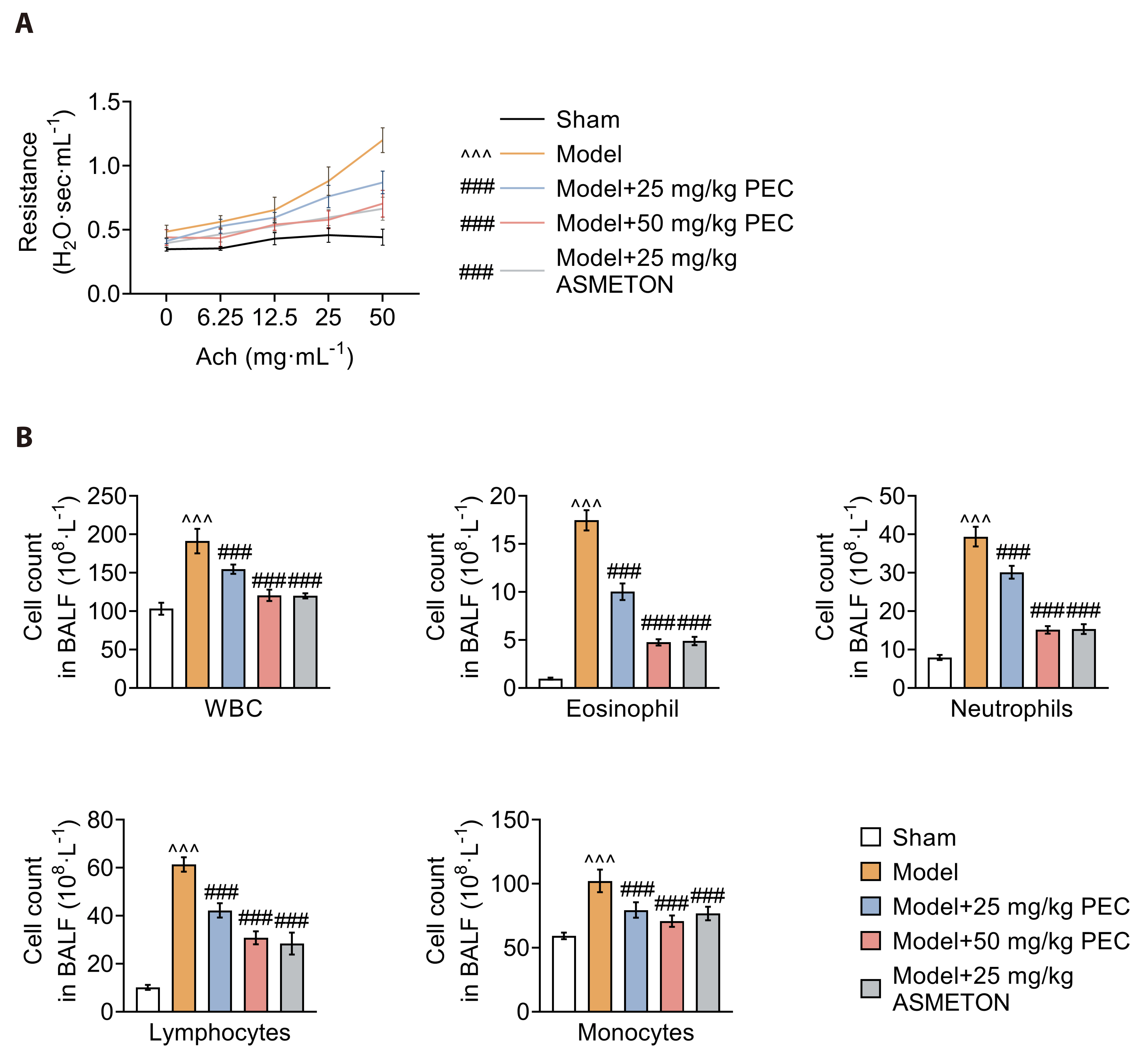
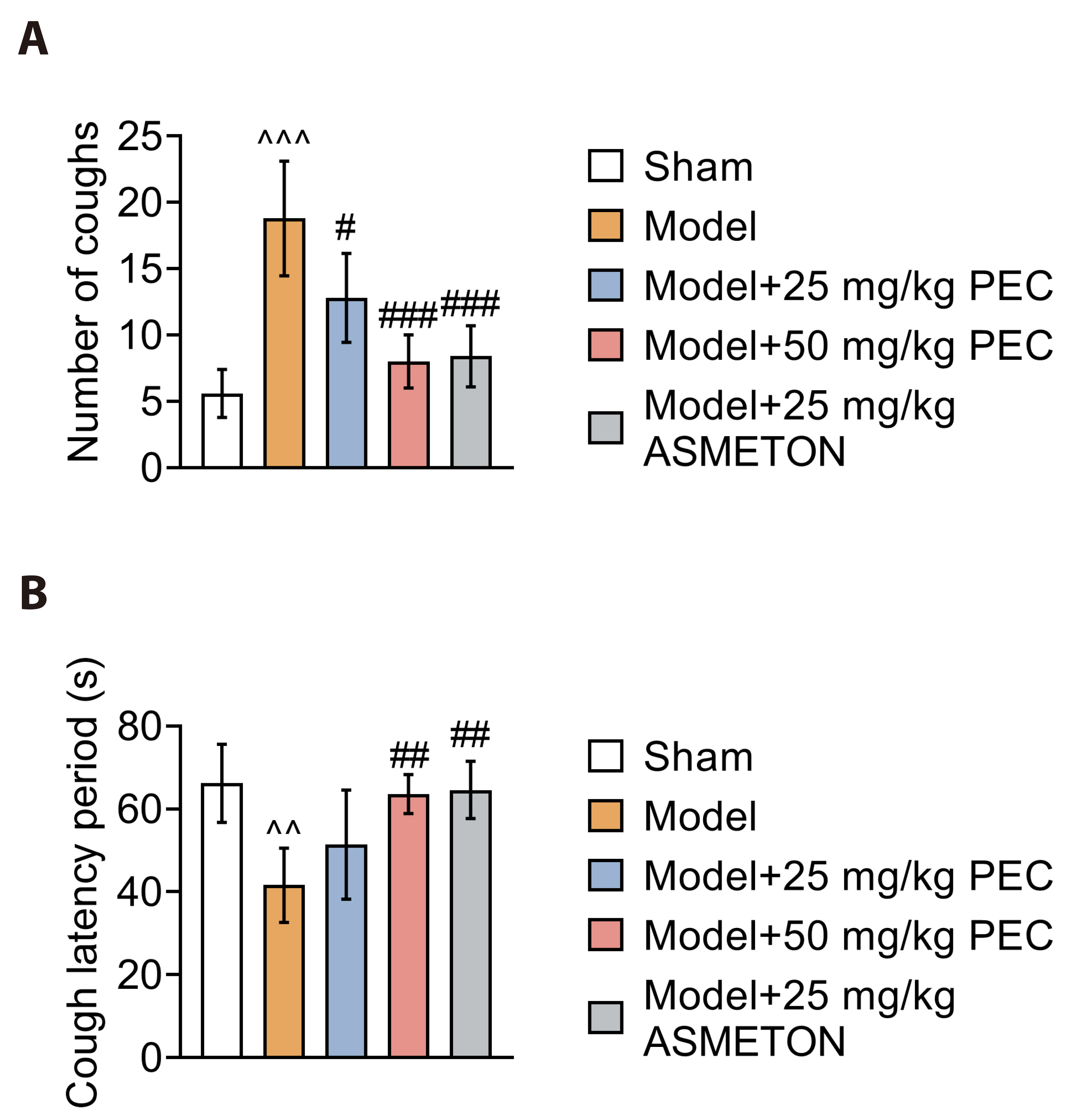
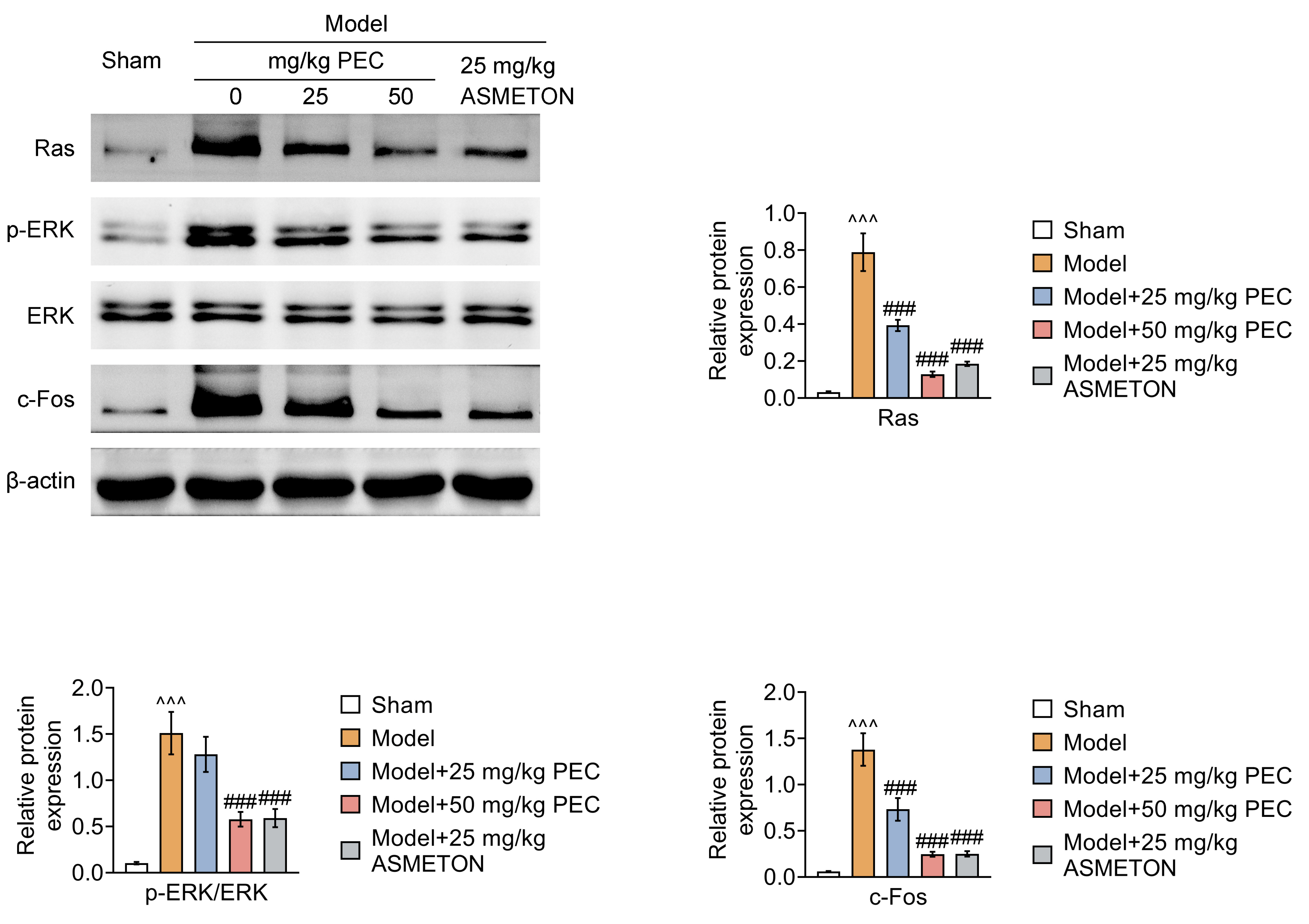
- Cough is a common symptom of several respiratory diseases. However, frequent coughing from acute to chronic often causes great pain to patients. It may turn into cough variant asthma, which seriously affects people's quality of life. For cough treatment, it is dominated by over-the-counter antitussive drugs, such as asmeton, but most currently available antitussive drugs have serious side effects. Thus, there is a great need for the development of new drugs with potent cough suppressant. BALB/c mice were used to construct mice model with cough to investigate the pharmacological effects of pectolinarigenin (PEC). Hematoxylin-eosin and Masson staining were used to assess lung injury and airway remodeling, and ELISA was used to assess the level of inflammatory factor release. In addition, inflammatory cell counts were measured to assess airway inflammation. Airway hyperresponsiveness assay was used to assess respiratory resistance in mice. Finally, we used Western blotting to explore the potential mechanisms of PEC. We found that PEC could alleviate lung tissue injury and reduce the release of inflammatory factors, inhibit of cough frequency and airway wall collagen deposition in mice model with cough. Meanwhile, PEC inhibited the Ras/ERK/c-Fos pathway to exhibit antitussive effect. Therefore, PEC may be a potential drug for cough suppression.
Keyword
Figure
Reference
-
1. Gibson PG. 2019; Chronic cough. J Allergy Clin Immunol Pract. 7:1762. DOI: 10.1016/j.jaip.2019.05.012. PMID: 31279463.
Article2. Chamberlain SA, Garrod R, Douiri A, Masefield S, Powell P, Bücher C, Pandyan A, Morice AH, Birring SS. 2015; The impact of chronic cough: a cross-sectional European survey. Lung. 193:401–408. Erratum in: Lung. 2015;193:615. DOI: 10.1007/s00408-015-9701-2. PMID: 25787221.
Article3. Zhai R, Lenga Ma Bonda W, Matute-Bello G, Jabaudon M. 2022; From preclinical to clinical models of acute respiratory distress syndrome. Signa Vitae. 18:3–14.4. Hossain SS, Islam MS, Rahman MM, De S, Mahmud K. 2016; Clinical and demographic profiles of patients diagnosed as cough variant asthma attended at tertiary referral hospital. J Natl Inst Neurosci Bangladesh. 2:30–33. DOI: 10.3329/jninb.v2i1.32960.
Article5. Uryasjev MO, Ponomareva IV, Bhar M, Glotov SI. 2020; [The cough variant asthma]. Ter Arkh. 92:98–101. Russian. DOI: 10.26442/00403660.2020.03.000404. PMID: 32598800.
Article6. Ding H, Shi C, Xu X, Yu L. 2020; Drug-induced chronic cough and the possible mechanism of action. Ann Palliat Med. 9:3562–3570. DOI: 10.21037/apm-20-819. PMID: 32921095.
Article7. Wang P, Shang E, Fan X. 2021; Effect of San'ao decoction with scorpio and bombyx batryticatus on CVA mice model via airway inflammation and regulation of TRPA1/TRPV1/TRPV5 channels. J Ethnopharmacol. 264:113342. DOI: 10.1016/j.jep.2020.113342. PMID: 32890712.
Article8. Wen L, Zhang T, Chen F, Hu L, Dou C, Ding X, Altamirano A, Wei G, Yan Z. 2023; Modified Dingchuan Decoction treats cough-variant asthma by suppressing lung inflammation and regulating the lung microbiota. J Ethnopharmacol. 306:116171. Erratum in: J Ethnopharmacol. 2024;318:116752. DOI: 10.1016/j.jep.2023.116752. PMID: 37328331.
Article9. Tan Z, Liu Q, Chen H, Zhang Z, Wang Q, Mu Y, Li Y, Hu T, Yang Y, Yan X. 2023; Pectolinarigenin alleviated septic acute kidney injury via inhibiting Jak2/Stat3 signaling and mitochondria dysfunction. Biomed Pharmacother. 159:114286. DOI: 10.1016/j.biopha.2023.114286. PMID: 36706631.
Article10. Ren Q, Wang B, Guo F, Huang R, Tan Z, Ma L, Fu P. 2022; Natural flavonoid pectolinarigenin alleviated hyperuricemic nephropathy via suppressing TGFβ/SMAD3 and JAK2/STAT3 signaling pathways. Front Pharmacol. 12:792139. DOI: 10.3389/fphar.2021.792139. PMID: 35153751. PMCID: PMC8829971.
Article11. Feng Y, Bhandari R, Li C, Shu P, Shaikh II. 2022; Pectolinarigenin suppresses LPS-induced inflammatory response in macrophages and attenuates DSS-induced colitis by modulating the NF-κB/Nrf2 signaling pathway. Inflammation. 45:2529–2543. DOI: 10.1007/s10753-022-01710-4. PMID: 35931839.
Article12. He Q, Liu C, Shen L, Zeng L, Wang T, Sun J, Zhou X, Wan J. 2021; Theory of the exterior-interior relationship between the lungs and the large intestine to explore the mechanism of Eriobotrya japonica leaf water extract in the treatment of cough variant asthma. J Ethnopharmacol. 281:114482. DOI: 10.1016/j.jep.2021.114482. PMID: 34438032.
Article13. O'Byrne PM, Inman MD. 2003; Airway hyperresponsiveness. Chest. 123(3 Suppl):411S–416S. DOI: 10.1378/chest.123.3_suppl.411S. PMID: 12629006.14. Wang W, Luo X, Zhang Q, He X, Zhang Z, Wang X. 2020; Bifidobacterium infantis relieves allergic asthma in mice by regulating Th1/Th2. Med Sci Monit. 26:e920583. DOI: 10.12659/MSM.920583.
Article15. Lu S, Guo M, Fan Z, Chen Y, Shi X, Gu C, Yang Y. 2019; Elevated TRIP13 drives cell proliferation and drug resistance in bladder cancer. Am J Transl Res. 11:4397–4410.16. Mousa AM, Almatroudi A, Alwashmi AS, Abdulmonem WA, Aljohani ASM, Alhumaydhi FA, Alsahli MA, Alrumaihi F, Allemailem KS, Abdellatif AAH, Khan A, Khan MA, Alshabrmi FM, Alruwetei A, Aljasir M, Aba Alkhayl FF, Rahmani AH, Rugaie OA, Alnuqaydan AM, Alsagaby SA, et al. 2021; Thyme oil alleviates Ova-induced bronchial asthma through modulating Th2 cytokines, IgE, TSLP and ROS. Biomed Pharmacother. 140:111726. DOI: 10.1016/j.biopha.2021.111726. PMID: 34111725.
Article17. Bao ZS, Hong L, Guan Y, Dong XW, Zheng HS, Tan GL, Xie QM. 2011; Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int Immunopharmacol. 11:899–906. DOI: 10.1016/j.intimp.2011.02.001. PMID: 21354484.
Article18. Xie M, Liu T, Yin J, Liu J, Yang L, Li T, Xia C, Fan Y. 2024; Kechuanning gel plaster exerts anti-inflammatory and immunomodulatory effects on ovalbumin-induced asthma model rats via ERK pathway. Comb Chem High Throughput Screen. 27:69–77. DOI: 10.2174/1386207326666230503105935. PMID: 37138476.
Article19. Sobczak Ł, Goryński K. 2020; Pharmacological aspects of over-the-counter opioid drugs misuse. Molecules. 25:3905. DOI: 10.3390/molecules25173905. PMID: 32867117. PMCID: PMC7504308.
Article20. Roy SD, Hawes EM, Hubbard JW, McKay G, Midha KK. 1984; Methoxyphenamine and dextromethorphan as safe probes for debrisoquine hydroxylation polymorphism. Lancet. 2:1393. DOI: 10.1016/S0140-6736(84)92081-6. PMID: 6150386.
Article21. Hu JR, Jung CJ, Ku SM, Jung DH, Ku SK, Choi JS. 2019; Antitussive, expectorant, and anti-inflammatory effects of Adenophorae Radix powder in ICR mice. J Ethnopharmacol. 239:111915. DOI: 10.1016/j.jep.2019.111915. PMID: 31039428.
Article22. Tang J, Ji H, Shi J, Wu L. 2016; Ephedra water decoction and cough tablets containing ephedra and liquorice induce CYP1A2 but not CYP2E1 hepatic enzymes in rats. Xenobiotica. 46:141–146. DOI: 10.3109/00498254.2015.1060371. PMID: 26153439.
Article23. Zhang B, Zeng M, Zhang Q, Wang R, Jia J, Cao B, Liu M, Guo P, Zhang Y, Zheng X, Feng W. 2022; Ephedrae Herba polysaccharides inhibit the inflammation of ovalbumin induced asthma by regulating Th1/Th2 and Th17/Treg cell immune imbalance. Mol Immunol. 152:14–26. DOI: 10.1016/j.molimm.2022.09.009. PMID: 36215828.
Article24. Ji K, Ma JL, Shi LQ, Wang LM, Li NN, Dong SJ, Lin B, Wang LY, Wen SH. 2023; Effects of qufeng xuanfei formula in guinea pig model of airway hyperergy. Pak J Pharm Sci. 36:205–210.25. Callaway JK, King RG, Boura AL. 1990; Methoxyphenamine inhibits histamine-induced bronchoconstriction in anaesthetized guinea-pigs and histamine-induced contractions of guinea-pig ileum in vitro. Arch Int Pharmacodyn Ther. 308:86–94.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evolution of Asthma Concept and Effect of Current Asthma Management Guidelines
- Airway epithelial cells in airway inflammation and remodeling in asthma
- Effects of CpG-oligodeoxynucleotides in Chronic Inflammation and Remodeling of Airway in a Murine Model of Bronchial Asthma
- Mechanical Stimuli to Airway Remodeling in Asthma
- Bronchial Structural Changes in Childhood Asthma