Cancer Res Treat.
2024 Apr;56(2):590-601. 10.4143/crt.2023.1117.
A Phase 1b/2a Study of GC1118 with 5-Fluorouracil, Leucovorin and Irinotecan (FOLFIRI) in Patients with Recurrent or Metastatic Colorectal Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Seoul National University Cancer Research Institute, Seoul, Korea
- 4Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 6Center for Colorectal Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 7Division of Hemato-Oncology, Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- 8Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 9Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- 10GC Biopharma Corp., Yongin, Korea
- KMID: 2554348
- DOI: http://doi.org/10.4143/crt.2023.1117
Abstract
- Purpose
GC1118 is a novel antibody targeting epidermal growth factor receptor (EGFR) with enhanced blocking activity against both low- and high-affinity EGFR ligands. A phase 1b/2a study was conducted to determine a recommended phase 2 dose (RP2D) of GC1118 in combination with 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) (phase 1b) and to assess the safety and efficacy of GC1118 plus FOLFIRI as a second-line therapy for recurrent/metastatic colorectal cancer (CRC) (phase 2a).
Materials and Methods
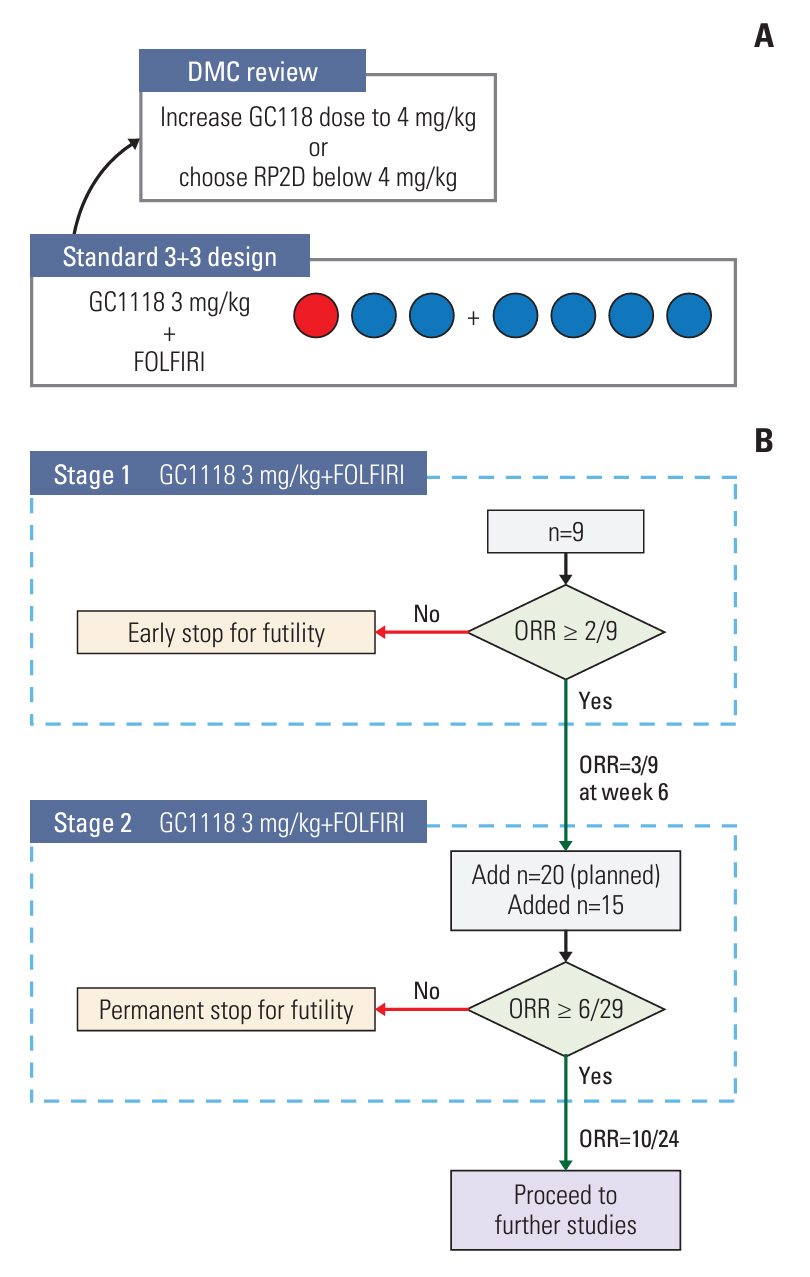
Phase 1b was designed as a standard 3+3 dose-escalation study with a starting dose of GC1118 (3 mg/kg/week) in combination with biweekly FOLFIRI (irinotecan 180 mg/m2; leucovorin 400 mg/m2; 5-fluorouracil 400 mg/m2 bolus and 2,400 mg/m2 infusion over 46 hours) in patients with solid tumors refractory to standard treatments. The subsequent phase 2a part was conducted with objective response rate (ORR) as a primary endpoint. Patients with KRAS/NRAS/BRAF wild-type, EGFR-positive, recurrent/metastatic CRC resistant to the first-line treatment were enrolled in the phase 2a study.
Results
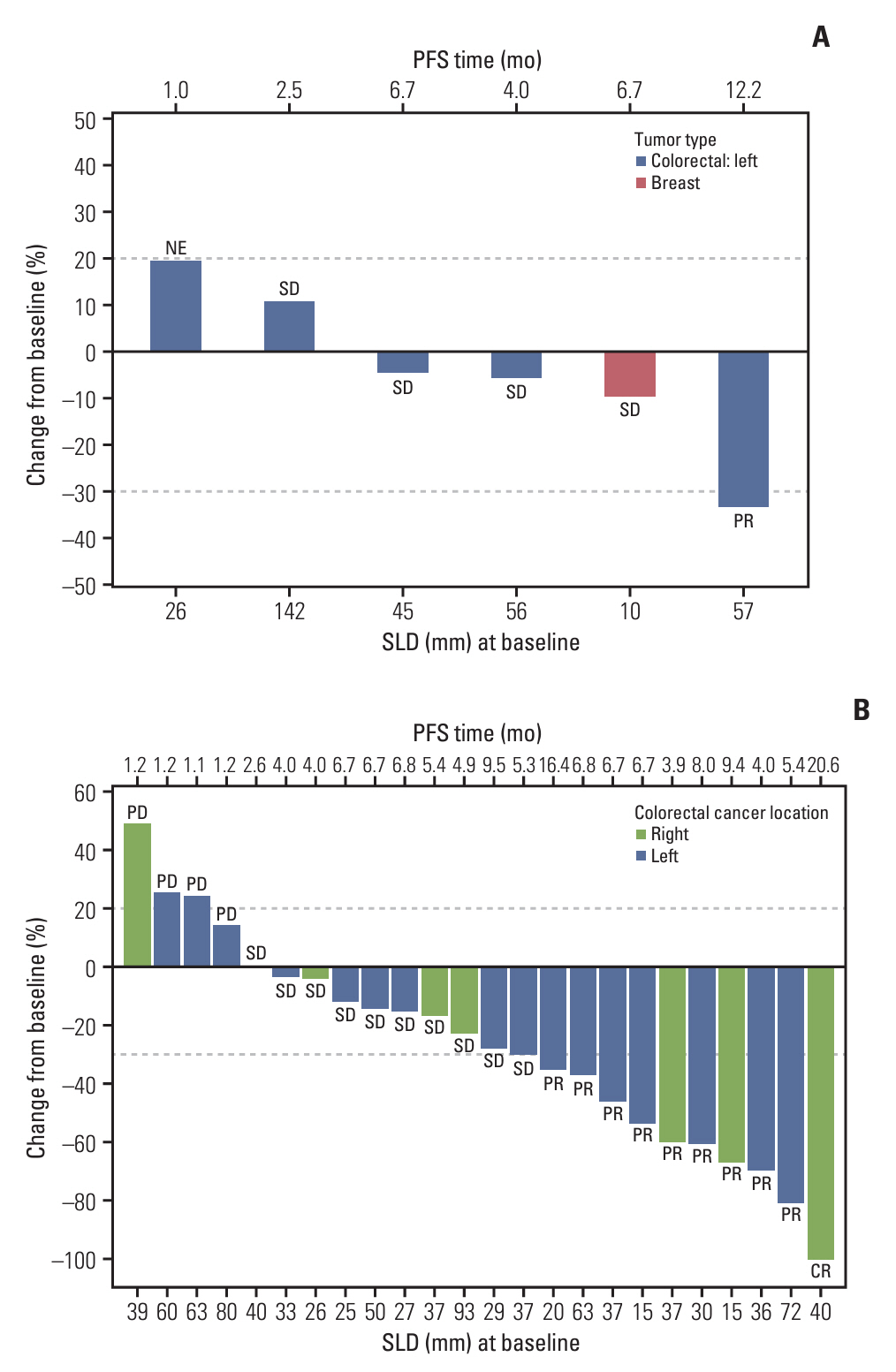
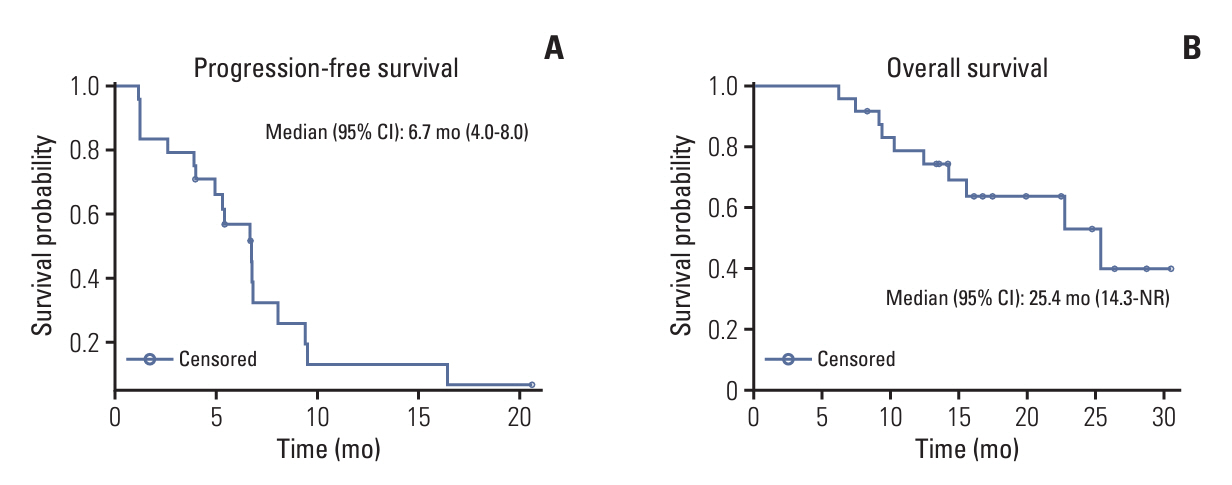
RP2D of GC1118 was determined to be 3 mg/kg/wk in the phase 1b study (n=7). Common adverse drug reactions (ADRs) observed in the phase 2a study (n=24) were acneiform rash (95.8%), dry skin (66.7%), paronychia (58.3%), and stomatitis (50.0%). The most common ADR of ≥ grade 3 was neutropenia (33.3%). ORR was 42.5% (95% confidence interval [CI], 23.5 to 62.0), and median progression-free survival was 6.7 months (95% CI, 4.0-8.0).
Conclusion
GC1118 administered weekly at 3 mg/kg in combination with FOLFIRI appears as an effective and safe treatment option in recurrent/metastatic CRC.
Figure
Reference
-
References
1. Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, et al. Colorectal cancer epidemiology: recent trends and impact on outcomes. Curr Drug Targets. 2021; 22:998–1009.
Article2. Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021; 149:778–89.
Article3. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021; 325:669–85.
Article4. Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009; 158:1–9.
Article5. Yewale C, Baradia D, Vhora I, Patil S, Misra A. Epidermal growth factor receptor targeting in cancer: a review of trends and strategies. Biomaterials. 2013; 34:8690–707.
Article6. Messersmith WA, Ahnen DJ. Targeting EGFR in colorectal cancer. N Engl J Med. 2008; 359:1834–6.
Article7. Bronte G, Silvestris N, Castiglia M, Galvano A, Passiglia F, Sortino G, et al. New findings on primary and acquired resistance to anti-EGFR therapy in metastatic colorectal cancer: do all roads lead to RAS? Oncotarget. 2015; 6:24780–96.
Article8. Zhou J, Ji Q, Li Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: underlying mechanisms and reversal strategies. J Exp Clin Cancer Res. 2021; 40:328.
Article9. Lim Y, Yoo J, Kim MS, Hur M, Lee EH, Hur HS, et al. GC1118, an Anti-EGFR antibody with a distinct binding epitope and superior inhibitory activity against high-affinity EGFR ligands. Mol Cancer Ther. 2016; 15:251–63.
Article10. Park WS, Han S, Lee J, Hong T, Won J, Lim Y, et al. Use of a target-mediated drug disposition model to predict the human pharmacokinetics and target occupancy of GC1118, an antiepidermal growth factor receptor antibody. Basic Clin Pharmacol Toxicol. 2017; 120:243–9.11. Park JE, Jin MH, Hur M, Nam AR, Bang JH, Won J, et al. GC1118, a novel anti-EGFR antibody, has potent KRAS mutation-independent antitumor activity compared with cetuximab in gastric cancer. Gastric Cancer. 2019; 22:932–40.
Article12. Lee HW, Son E, Lee K, Lee Y, Kim Y, Lee JC, et al. Promising therapeutic efficacy of GC1118, an anti-EGFR antibody, against KRAS mutation-driven colorectal cancer patient-derived xenografts. Int J Mol Sci. 2019; 20:5894.
Article13. Lee K, Koo H, Kim Y, Kim D, Son E, Yang H, et al. Therapeutic efficacy of GC1118, a novel anti-EGFR antibody, against glioblastoma with high EGFR amplification in patient-derived xenografts. Cancers (Basel). 2020; 12:3210.
Article14. Oh DY, Lee KW, Han SW, Kim JW, Shin JW, Jo SJ, et al. A first-in-human phase I study of GC1118, a novel anti-epidermal growth factor receptor antibody, in patients with advanced solid tumors. Oncologist. 2019; 24:1037–e636.
Article15. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989; 10:1–10.
Article16. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010; 28:4706–13.
Article17. Hecht JR, Cohn A, Dakhil S, Saleh M, Piperdi B, Cline-Burkhardt M, et al. SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin Colorectal Cancer. 2015; 14:72–80.
Article18. Chung TK, Lee HA, Park SI, Oh DY, Lee KW, Kim JW, et al. A target-mediated drug disposition population pharmacokinetic model of GC1118, a novel anti-EGFR antibody, in patients with solid tumors. Clin Transl Sci. 2021; 14:990–1001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Irinotecan, Continuous 5-Fluorouracil, and Low dose of Leucovorin (modified FOLFIRI) as First Line of Therapy in Recurrent or Metastatic Colorectal Cancer
- A Case of Organizing Pneumonia Associated with FOLFIRI Chemotherapy
- A Phase II Study of Irinotecan, 5-Fluorouracil and Leucovorin for Treatment in Patients with Previously Untreated Advanced Colorectal Cancer
- Effect of an Irinotencan, 5-Fluorouracil, and Leucovorin Combination Chemotherapy (FOLFIRI) in Metastatic Colorectal Cancer
- Combination chemotherapy of irinotecan combined with bolus 5-fluorouracil, continuous infusion 5-fluorouracil, and high dose leucovorin every two weeks in recurrent or metastatic colorectal cancer