J Korean Med Sci.
2023 Jul;38(27):e205. 10.3346/jkms.2023.38.e205.
Loss of Neutralizing Activity of Tixagevimab/Cilgavimab (Evusheld™) Against Omicron BN.1, a Dominant Circulating Strain Following BA.5 During the Seventh Domestic Outbreak in Korea in Early 2023
- Affiliations
-
- 1Division of Infectious Diseases, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Vaccine Development Coordination, Center for Vaccine Research, National Institute of Infectious Diseases, National Institute of Health, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 3Asia Pacific Foundation for Infectious Diseases (APFID), Seoul, Korea
- KMID: 2544411
- DOI: http://doi.org/10.3346/jkms.2023.38.e205
Abstract
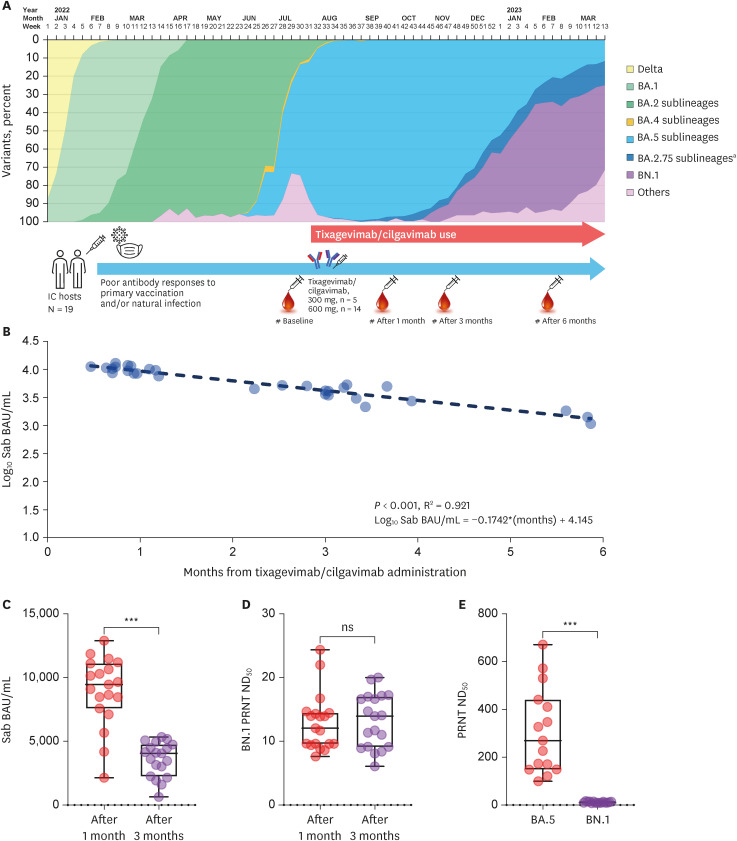
- Tixagevimab/cilgavimab is a monoclonal antibody used to prevent coronavirus disease 2019 among immunocompromised hosts and maintained neutralizing activity against early omicron variants. Omicron BN.1 became a dominant circulating strain in Korea early 2023, but its susceptibility to tixagevimab/cilgavimab is unclear. We conducted plaque reduction neutralization test (PRNT) against BN.1 in a prospective cohort (14 patients and 30 specimens). BN.1 PRNT was conducted for one- and three-months after tixagevimab/ cilgavimab administration and the average PRNT ND 50 of each point was lower than the positive cut-off value of 20 (12.9 ± 4.5 and 13.2 ± 4.2, respectively, P = 0.825). In the paired analyses, tixagevimab/cilgavimab-administered sera could not actively neutralize BN.1 (PRNT ND 50 11.5 ± 2.9, P = 0.001), compared with the reserved activity against BA.5 (ND 50 310.5 ± 180.4). Unlike virus-like particle assay, tixagevimab/cilgavimab was not active against BN.1 in neutralizing assay, and would not be effective in the present predominance of BA.2.75 sublineages.
Keyword
Figure
Reference
-
1. Yang J, Won G, Baek JY, Lee YH, Kim H, Huh K, et al. Neutralizing activity against omicron BA.5 after tixagevimab/cilgavimab administration comparable to those after omicron BA.1/BA.2 breakthrough infections. Front Immunol. 2023; 14:1139980. PMID: 36936968.2. World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Updated 2021. Accessed on April 10, 2023. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern .3. Lee JJ, Choe YJ, Jeong H, Kim M, Kim S, Yoo H, et al. Importation and transmission of SARS-CoV-2 B.1.1.529 (omicron) variant of concern in Korea, November 2021. J Korean Med Sci. 2021; 36(50):e346. PMID: 34962117.4. Callaway E. COVID ‘variant soup’ is making winter surges hard to predict. Updated 2022. Accessed on April 10, 2023. https://www.nature.com/articles/d41586-022-03445-6 .5. Ahn S, Jang J, Park SY, Yang S, Ryu B, Shin E, et al. Outbreak report of COVID-19 during designation of class 1 infectious disease in the Republic of Korea (January 20, 2020–April 24, 2022). Public Health Wkly Rep. 2022; 15(30):2126–2136.6. U.S. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Evusheld™ (tixagevimab co-packaged with cilgavimab). Updated 2023. Accessed April 28, 2023. https://www.fda.gov/media/154701/download .7. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018; 126(5):1763–1768. PMID: 29481436.8. National Institutes of Health. Anti-SARS-CoV-2 monoclonal antibodies, COVID-19 treatment guidelines. Updated 2023. Accessed on April 10, 2023. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/anti-sars-cov-2-monoclonal-antibodies/ .9. Lee JY, Lee JY, Ko JH, Hyun M, Kim HA, Cho S, et al. Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease. Front Immunol. 2021; 12:772320. PMID: 34899724.10. Park S, Je NK, Kim DW, Park M, Heo J. Effectiveness and safety of regdanvimab in patients with mild-to-moderate COVID-19: a retrospective cohort study. J Korean Med Sci. 2022; 37(13):e102. PMID: 35380027.11. Loo YM, McTamney PM, Arends RH, Abram ME, Aksyuk AA, Diallo S, et al. The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans. Sci Transl Med. 2022; 14(635):eabl8124. PMID: 35076282.12. Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19. N Engl J Med. 2022; 386(23):2188–2200. PMID: 35443106.13. Montgomery H, Hobbs FDR, Padilla F, Arbetter D, Templeton A, Seegobin S, et al. Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2022; 10(10):985–996. PMID: 35688164.14. Oh YJ, Kim J, Kang ES, Rhu J, Choi GS, Joh JW. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients: a single-center study. J Korean Med Sci. 2023; 38(16):e121. PMID: 37096307.15. Yang J, Lee KW, Baek JY, Bae S, Lee YH, Kim H, et al. Augmented humoral and cellular immunity against severe acute respiratory syndrome coronavirus 2 after breakthrough infection in kidney transplant recipients who received 3 doses of coronavirus disease 2019 vaccine. Am J Transplant. 2023; 23(4):565–572. PMID: 36739177.16. Song SH, Chung KY, Jee Y, Chung HS, Kim K, Minn D, et al. Immunogenicity of SARS-CoV-2 vaccine in kidney transplant recipients: a cross-sectional study in Korea. J Korean Med Sci. 2023; 38(5):e22. PMID: 36747360.17. Planas D, Bruel T, Staropoli I, Guivel-Benhassine F, Porrot F, Maes P, et al. Resistance of omicron subvariants BA.2.75.2, BA.4.6, and BQ.1.1 to neutralizing antibodies. Nat Commun. 2023; 14(1):824. PMID: 36788246.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Neutralizing Activities to the Omicron Subvariants BN.1 and XBB.1.5 of Sera From the Individuals Vaccinated With a BA.4/5-Containing Bivalent mRNA Vaccine
- A Model-Based Cost-Effectiveness Analysis of Long-Acting Monoclonal Antibody (Tixagevimab and Cilgavimab: Evusheld) Preventive Treatment for High-Risk Populations Against SARSCoV-2 in Korea
- Neutralizing Activity and T-Cell Responses Against Wild Type SARSCoV-2 Virus and Omicron BA.5 Variant After Ancestral SARS-CoV-2 Vaccine Booster Dose in PLWH Receiving ART Based on CD4 T-Cell Count
- Effect of Paxlovid in COVID-19 treatment during the periods of SARS-CoV-2 Omicron BA.5 and BN.1 subvariant dominance in the Republic of Korea: a retrospective cohort study
- Neutralization Testing–based Immunogenicity Analysis of Recent Prevalent Severe Acute Respiratory Syndrome Coronavirus 2 Omicron Sublineages