J Korean Med Sci.
2025 Mar;40(9):e28. 10.3346/jkms.2025.40.e28.
Neutralizing Activity and T-Cell Responses Against Wild Type SARSCoV-2 Virus and Omicron BA.5 Variant After Ancestral SARS-CoV-2 Vaccine Booster Dose in PLWH Receiving ART Based on CD4 T-Cell Count
- Affiliations
-
- 1Emerging Infectious Diseases Research Institute, Chungnam National University Hospital, Daejeon, Korea
- 2Translational Immunology Institute, Chungnam National University, Daejeon, Korea
- 3Division of Clinical Research for Vaccine, Center for Vaccine Research, Korea National Institute of Infectious Diseases, Cheongju, Korea
- 4Division of Infectious Diseases, Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea
- 5Department of Health Care Policy Research, Korea Institute for Health and Social Affairs, Sejong, Korea
- KMID: 2565799
- DOI: http://doi.org/10.3346/jkms.2025.40.e28
Abstract
- Background
We evaluated severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2)-specific humoral and cellular responses for up to 6 months after the 3rd dose of ancestral coronavirus disease 2019 (COVID-19) vaccination in people living with HIV (PLWH) and healthy controls (HCs) who were not infected with COVID-19.
Methods
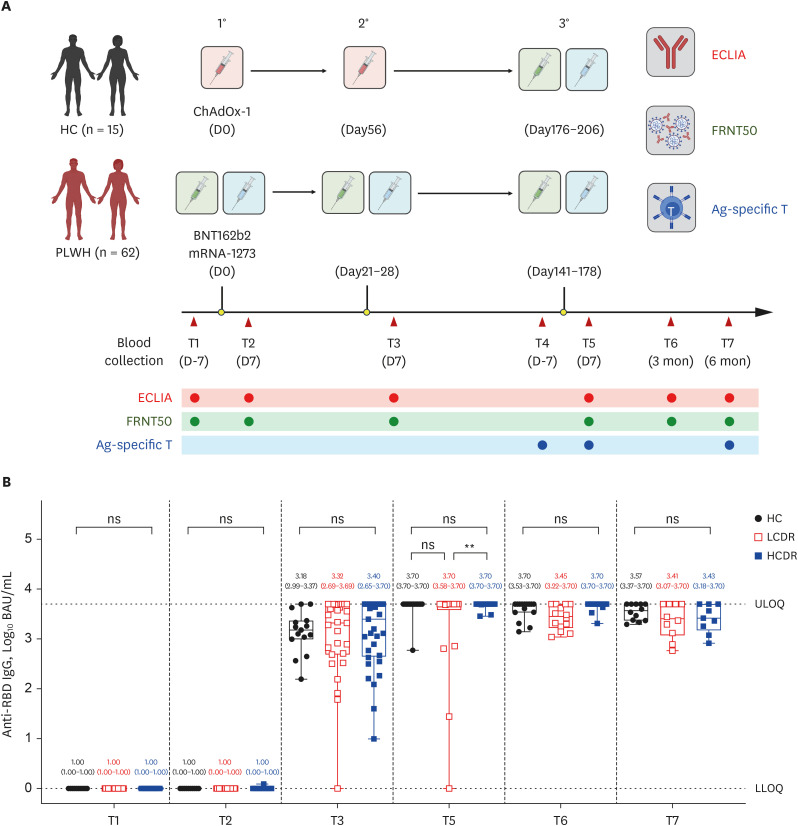
Anti-spike receptor-binding domain IgG (anti-RBD IgG) concentrations using chemiluminescence immunoassay and neutralizing antibodies using focus reduction neutralization test (FRNT) were assessed at 1 week after each dose of vaccination, and 3 and 6 months after the 3rd dose in 62 PLWH and 25 HCs. T-cell responses using intracellular cytokine stain were evaluated at 1 week before, and 1 week and 6 months after the 3rd dose.
Results
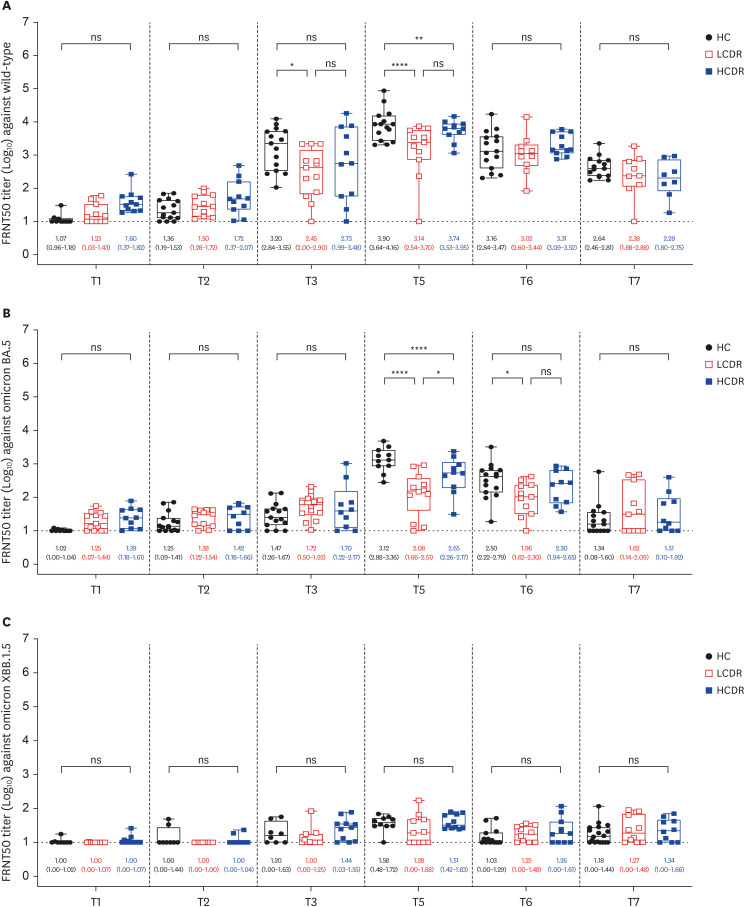
At 1 week after the 3rd dose, adequate anti-RBD IgG (> 300 binding antibody unit /mL) was elicited in all PLWH except for one patient with 36 CD4 T-cell count/mm3 . The geometric mean titers of 50% FRNT against wild type (WT) and omicron BA.5 strains of SARS-CoV-2 in PLWH with CD4 T-cell count ≥ 500 cells/mm3(high CD4 recovery, HCDR) were comparable to HC, but they were significantly decreased in PLWH with CD4 T-cell count < 500/mm3 (low CD4 recovery, LCDR). After adjusting for age, gender, viral suppression, and number of preexisting comorbidities, CD4 T-cell counts < 500/mm3 significantly predicted a poor magnitude of neutralizing antibodies against WT, omicron BA.5, and XBB 1.5 strains among PLWH. Multivariable linear regression adjusting for age and gender revealed that LCDR was associated with reduced neutralizing activity (P = 0.017) and interferon-γ-producing T-cell responses (P = 0.049 for CD T-cell; P = 0.014 for CD8 T-cell) against WT, and strongly associated with more decreased cross-neutralization against omicron BA.5 strains (P < 0.001).
Conclusion
HCDR demonstrated robust humoral and cell-mediated immune responses after a booster dose of ancestral SARS-CoV-2 vaccine, whereas LCDR showed diminished immune responses against WT virus and more impaired cross-neutralization against omicron BA.5 strain.
Figure
Reference
-
1. Kernéis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boëlle PY. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014; 58(8):1130–1139. PMID: 24415637.2. Parmigiani A, Alcaide ML, Freguja R, Pallikkuth S, Frasca D, Fischl MA, et al. Impaired antibody response to influenza vaccine in HIV-infected and uninfected aging women is associated with immune activation and inflammation. PLoS One. 2013; 8(11):e79816. PMID: 24236161.3. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011; 62(1):141–155. PMID: 21090961.4. Danwang C, Noubiap JJ, Robert A, Yombi JC. Outcomes of patients with HIV and COVID-19 co-infection: a systematic review and meta-analysis. AIDS Res Ther. 2022; 19(1):3. PMID: 35031068.5. Ambrosioni J, Blanco JL, Reyes-Urueña JM, Davies MA, Sued O, Marcos MA, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021; 8(5):e294–e305. PMID: 33915101.6. Geretti AM, Stockdale AJ, Kelly SH, Cevik M, Collins S, Waters L, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis. 2021; 73(7):e2095–e2106. PMID: 33095853.7. Bertagnolio S, Thwin SS, Silva R, Nagarajan S, Jassat W, Fowler R, et al. Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV. 2022; 9(7):e486–e495. PMID: 35561704.8. World Health Organization. Coronavirus disease (COVID-19) and people living with HIV. Updated 2023. Accessed May 15, 2024. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-covid-19-and-people-living-with-hiv .9. Levy I, Wieder-Finesod A, Litchevsky V, Biber A, Indenbaum V, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021; 27(12):1851–1855. PMID: 34438069.10. Brumme ZL, Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Duncan MC, et al. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines. 2022; 7(1):28. PMID: 35228535.11. Costiniuk CT, Singer J, Lee T, Langlois MA, Arnold C, Galipeau Y, et al. COVID-19 vaccine immunogenicity in people with HIV. AIDS. 2023; 37(1):F1–F10. PMID: 36476452.12. Lapointe HR, Mwimanzi F, Cheung PK, Sang Y, Yaseen F, Speckmaier S, et al. Antibody response durability following three-dose coronavirus disease 2019 vaccination in people with HIV receiving suppressive antiretroviral therapy. AIDS. 2023; 37(5):709–721. PMID: 36545783.13. Noe S, Ochana N, Wiese C, Schabaz F, Von Krosigk A, Heldwein S, et al. Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection. 2022; 50(3):617–623. PMID: 34694595.14. Tau L, Turner D, Adler A, Marom R, Ahsanov S, Matus N, et al. SARS-CoV-2 humoral and cellular immune responses of patients with HIV after vaccination with BNT162b2 mRNA COVID-19 vaccine in the Tel-Aviv Medical Center. Open Forum Infect Dis. 2022; 9(4):ofac089. PMID: 35355894.15. Benet S, Blanch-Lombarte O, Ainsua-Enrich E, Pedreño-Lopez N, Muñoz-Basagoiti J, Raïch-Regué D, et al. Limited humoral and specific T-cell responses after SARS-CoV-2 vaccination in PLWH with poor immune reconstitution. J Infect Dis. 2022; 226(11):1913–1923. PMID: 36200261.16. Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A, et al. Humoral and cellular immune response elicited by mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in people living with human immunodeficiency virus receiving antiretroviral therapy based on current CD4 T-lymphocyte count. Clin Infect Dis. 2022; 75(1):e552–e563. PMID: 35366316.17. Vergori A, Cozzi Lepri A, Cicalini S, Matusali G, Bordoni V, Lanini S, et al. Immunogenicity to COVID-19 mRNA vaccine third dose in people living with HIV. Nat Commun. 2022; 13(1):4922. PMID: 35995780.18. Vergori A, Cozzi-Lepri A, Matusali G, Colavita F, Cicalini S, Gallì P, et al. SARS-CoV-2 omicron variant neutralization after third dose vaccination in PLWH. Viruses. 2022; 14(8):1710. PMID: 36016332.19. Tan ST, Kwan AT, Rodríguez-Barraquer I, Singer BJ, Park HJ, Lewnard JA, et al. Infectiousness of SARS-CoV-2 breakthrough infections and reinfections during the omicron wave. Nat Med. 2023; 29(2):358–365. PMID: 36593393.20. Ai J, Zhang H, Zhang Y, Lin K, Zhang Y, Wu J, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022; 11(1):337–343. PMID: 34935594.21. Corma-Gómez A, Fernández-Fuertes M, Viñuela L, Domínguez C, Santos M, Fuentes-López A, et al. Reduced neutralizing antibody response to SARS-CoV-2 vaccine booster dose in people living with HIV with severe immunosuppression. J Med Virol. 2023; 95(3):e28602. PMID: 36880164.22. Levy I, Rahav G. The effect of HIV on COVID-19 vaccine responses. Curr Opin HIV AIDS. 2023; 18(3):135–141. PMID: 36943427.23. Alrubayyi A, Gea-Mallorquí E, Touizer E, Hameiri-Bowen D, Kopycinski J, Charlton B, et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun. 2021; 12(1):5839. PMID: 34611163.24. Spinelli MA, Peluso MJ, Lynch KL, Yun C, Glidden DV, Henrich TJ, et al. Differences in post-mRNA vaccination SARSCoV-2 IgG concentrations and surrogate virus neutralization test response by HIV status and type of vaccine: a matched case-control observational study. Clin Infect Dis. 2022; 75(1):e916–e919. PMID: 34864962.25. Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021; 54(9):2133–2142.e3. PMID: 34453880.26. Datwani S, Kalikawe R, Waterworth R, Mwimanzi FM, Liang R, Sang Y, et al. T-cell responses to COVID-19 vaccines and breakthrough infection in people living with HIV receiving antiretroviral therapy. Viruses. 2024; 16(5):661. PMID: 38793543.27. Arunachalam PS, Lai L, Samaha H, Feng Y, Hu M, Hui HS, et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J Clin Invest. 2023; 133(10):e167955. PMID: 36951954.28. Pitiriga VC, Papamentzelopoulou M, Konstantinakou KE, Vasileiou IV, Konstantinidis AD, Spyrou NI, et al. Pronlonged SARS-CoV-2 T cell responses in a vaccinated COVID-19-naïve population. Vaccines (Basel). 2024; 12(3):270. PMID: 38543904.29. Reinscheid M, Luxenburger H, Karl V, Graeser A, Giese S, Ciminski K, et al. COVID-19 mRNA booster vaccine induces transient CD8+ T effector cell responses while conserving the memory pool for subsequent reactivation. Nat Commun. 2022; 13(1):4631. PMID: 35941157.30. Mentzer AJ, O’Connor D, Bibi S, Chelysheva I, Clutterbuck EA, Demissie T, et al. Human leukocyte antigen alleles associate with COVID-19 vaccine immunogenicity and risk of breakthrough infection. Nat Med. 2023; 29(1):147–157. PMID: 36228659.31. Dan J, da Silva Antunes R, Grifoni A, Weiskopf D, Crotty S, Sette A. Observations and perspectives on adaptive immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2022; 75(Suppl 1):S24–S29. PMID: 35441229.32. Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021; 184(4):861–880. PMID: 33497610.33. Macías J, Fernández-Fuertes M, Oliver N, Corma-Gómez A, Real LM, Pineda JA. Lower probability of persistence of total anti-SARS-CoV-2 antibodies after COVID-19 among people living with HIV. Clin Microbiol Infect. 2022; 28(5):755–756. PMID: 35150883.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low Neutralizing Activities to the Omicron Subvariants BN.1 and XBB.1.5 of Sera From the Individuals Vaccinated With a BA.4/5-Containing Bivalent mRNA Vaccine
- SARS-CoV-2 mRNA Vaccine Elicits Sustained T Cell Responses Against the Omicron Variant in Adolescents
- Changes in SARS-CoV-2 antibody titers 6 months after the booster dose of BNT162b2 COVID-19 vaccine among health care workers
- Recombinant proteins of spike protein of SARS-CoV-2 with the Omicron receptor-binding domain induce production of highly Omicron-specific neutralizing antibodies
- Effects of Omicron Infection and Changes in Serum Antibody Response to Wild-Type, Delta, and Omicron After a Booster Dose With BNT163b2 Vaccine in Korean Healthcare Workers