J Korean Med Sci.
2023 Apr;38(13):e103. 10.3346/jkms.2023.38.e103.
Effects of Omicron Infection and Changes in Serum Antibody Response to Wild-Type, Delta, and Omicron After a Booster Dose With BNT163b2 Vaccine in Korean Healthcare Workers
- Affiliations
-
- 1Division of Hematology-Oncology, Department of Medicine, Soonchunhyang University, Bucheon Hospital, Bucheon, Korea
- 2Division of Hematology-Oncology, Department of Medicine, Soonchunhyang University, Cheonan Hospital, Cheonan, Korea
- 3Division of Infectious Disease, Department of Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea
- 4Department of Medical Biotechnology, Soonchunhyang University, Asan, Korea
- 5Department of Biostatistics, Clinical Trial Center, Soonchunhyang University Bucheon Hospital, Bucheon, Korea
- KMID: 2541044
- DOI: http://doi.org/10.3346/jkms.2023.38.e103
Abstract
- Background
Although the primary vaccine coverage rate for coronavirus disease 2019 (COVID-19) in South Korea has exceeded 80%, the coronavirus continues to spread, with reports of a rapid decline in vaccine effectiveness. South Korea is administering booster shots despite concerns about the effectiveness of the existing vaccine.
Methods
Neutralizing antibody inhibition scores were evaluated in two cohorts after the booster dose. For the first cohort, neutralizing activity against the wild-type, delta, and omicron variants after the booster dose was evaluated. For the second cohort, we assessed the difference in neutralizing activity between the omicron infected and uninfected groups after booster vaccination. We also compared the effectiveness and adverse events (AEs) between homologous and heterologous booster doses for BNT162b2 or ChAdOx1 vaccines.
Results
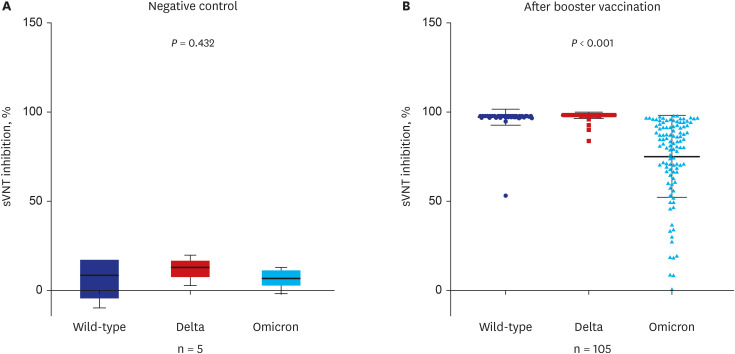
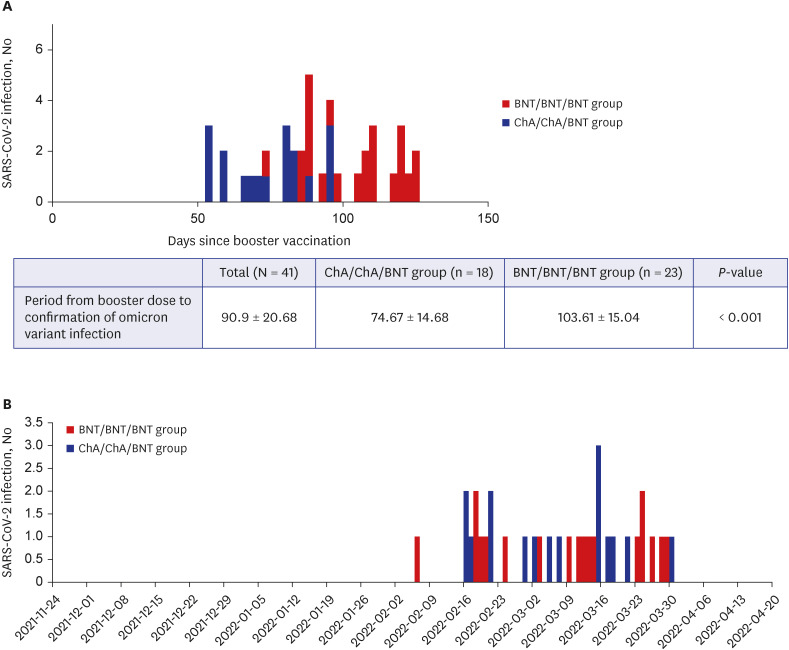
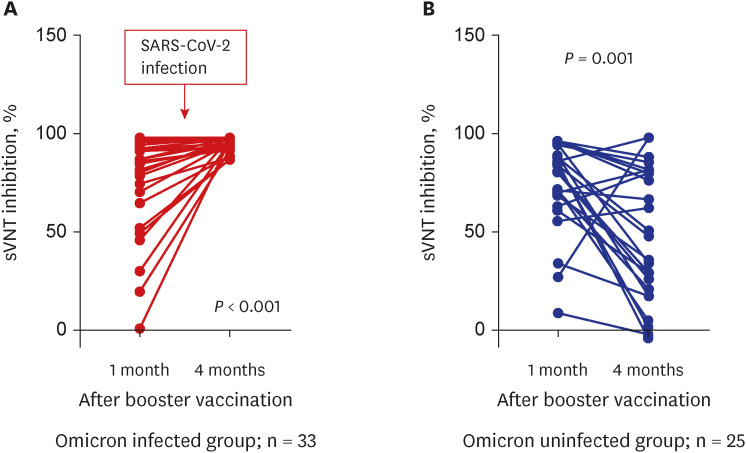
A total of 105 healthcare workers (HCWs) that were additionally vaccinated with BNT162b2 at Soonchunhyang University Bucheon Hospital were enrolled in this study. Significantly higher surrogate virus neutralization test (sVNT) inhibition (%) was observed for the wild-type and delta variants compared to sVNT (%) for the omicron after the booster dose (97%, 98% vs. 75%; P < 0.001). No significant difference in the neutralizing antibody inhibition score was found between variants in the BNT/BNT/BNT group (n = 48) and the ChA/ChA/BNT group (n = 57). Total AEs were not significantly different between the ChA/ ChA/BNT group (85.96%) and the BNT/BNT group (95.83%; P = 0.11). In the second cohort with 58 HCWs, markedly higher sVNT inhibition to omicron was observed in the omicroninfected group (95.13%) compared to the uninfected group (mean of 48.44%; P < 0.001) after four months of the booster dose. In 41 HCWs (39.0%) infected with the omicron variant, no difference in immunogenicity, AEs, or effectiveness between homogeneous and heterogeneous boosters was observed.
Conclusion
Booster vaccination with BNT162b2 was significantly less effective for the neutralizing antibody responses to omicron variant compared to the wild-type or delta variant in healthy population. Humoral immunogenicity was sustained significantly high after 4 months of booster vaccine in the infected population after booster vaccination. Further studies are needed to understand the characteristics of immunogenicity in these populations.
Figure
Cited by 1 articles
-
Eight-Month Follow-up After the Third Dose of BNT162b2 Vaccine in Healthcare Workers: The Question of a Fourth Dose
Sung Hee Lim, Seong Hyeok Choi, Ji Youn Kim, Bora Kim, Han Jo Kim, Se Hyung Kim, Chan Kyu Kim, Seong Kyu Park, Jina Yun
J Korean Med Sci. 2023;38(18):e139. doi: 10.3346/jkms.2023.38.e139.
Reference
-
1. Lim SH, Choi SH, Kim B, Kim JY, Ji YS, Kim SH, et al. Serum antibody response comparison and adverse reaction analysis in healthcare workers vaccinated with the BNT162b2 or ChAdOx1 COVID-19 vaccine. Vaccines (Basel). 2021; 9(12):1379. PMID: 34960125.
Article2. Noor R, Shareen S, Billah M. COVID-19 vaccines: their effectiveness against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its emerging variants. Bull Natl Res Cent. 2022; 46(1):96. PMID: 35431535.3. Kim SR, Kang HJ, Jeong HR, Jang SY, Lee JE, Kim DE, et al. Relative effectiveness of COVID-19 vaccination in healthcare workers: 3-dose versus 2-dose vaccination. J Korean Med Sci. 2022; 37(35):e267. PMID: 36065651.
Article4. Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature. 2022; 602(7898):671–675. PMID: 35016199.5. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022; 386(16):1532–1546. PMID: 35249272.6. Yu J, Collier AY, Rowe M, Mardas F, Ventura JD, Wan H, et al. Neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. N Engl J Med. 2022; 386(16):1579–1580. PMID: 35294809.7. Regular briefing of Korea Disease Control and Prevention Agency (19-Jul-2022). Updated 2022. Accessed November 16, 2022. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&act=view&list_no=720171 .8. Herzberg J, Fischer B, Becher H, Becker AK, Honarpisheh H, Guraya SY, et al. Cellular and humoral immune response to a third dose of BNT162b2 COVID-19 vaccine – a prospective observational study. Front Immunol. 2022; 13:896151. PMID: 35844588.9. Park JY, Choi SH, Chung JW, Hwang MH, Kim MC. Systemic adverse events and use of antipyretics predict the neutralizing antibody positivity early after the first dose of ChAdOx1 coronavirus disease vaccine. J Clin Med. 2021; 10(13):2844. PMID: 34199053.10. Addetia A, Crawford KH, Dingens A, Zhu H, Roychoudhury P, Huang ML, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020; 58(11):e02107-20. PMID: 32826322.11. Suntronwong N, Kanokudom S, Auphimai C, Assawakosri S, Thongmee T, Vichaiwattana P, et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J Med Virol. 2022; 94(12):5713–5722. PMID: 35924475.
Article12. Hammerschmidt SI, Bosnjak B, Bernhardt G, Friedrichsen M, Ravens I, Dopfer-Jablonka A, et al. Neutralization of the SARS-CoV-2 delta variant after heterologous and homologous BNT162b2 or ChAdOx1 nCoV-19 vaccination. Cell Mol Immunol. 2021; 18(10):2455–2456. PMID: 34426672.13. Regular briefing of Korea Disease Control and Prevention Agency (12-Apr-2022). Updated 2022. Accessed November 16, 2022. https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=719266&cg_code=&act=view&nPage=49 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in SARS-CoV-2 antibody titers 6 months after the booster dose of BNT162b2 COVID-19 vaccine among health care workers
- Booster BNT162b2 COVID-19 Vaccination Increases Neutralizing Antibody Titers Against the SARS-CoV-2 Omicron Variant in Both Young and Elderly Adults
- Relative Effectiveness of COVID-19 Vaccination in Healthcare Workers: 3-Dose Versus 2-Dose Vaccination
- Vaccine Effectiveness Against Severe Disease and Death for Patients With COVID-19 During the Delta-Dominant and Omicron-Emerging Periods: A K-COVE Study
- SARS-CoV-2 Breakthrough Infection after mRNA-1273 Booster among CoronaVac-Vaccinated Healthcare Workers