J Korean Med Sci.
2023 Aug;38(32):e250. 10.3346/jkms.2023.38.e250.
A Model-Based Cost-Effectiveness Analysis of Long-Acting Monoclonal Antibody (Tixagevimab and Cilgavimab: Evusheld) Preventive Treatment for High-Risk Populations Against SARSCoV-2 in Korea
- Affiliations
-
- 1Department of Public Health Sciences, School of Medicine, University of Connecticut, Farmington, CT, USA
- 2Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 3Artificial Intelligence and Big-Data Convergence Center, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 4Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Korea
- KMID: 2545202
- DOI: http://doi.org/10.3346/jkms.2023.38.e250
Abstract
- Background
Tixagevimab and cilgavimab (Evusheld) administration is a recommended strategy for unvaccinated patients with immunocompromised conditions and severe allergic reaction conditions to protect high-risk individuals and control the coronavirus disease 2019 (COVID-19) epidemic. We estimated the cost-effectiveness of Evusheld in key risk populations: 1) immunocompromised (vaccinated/unvaccinated), 2) severe allergic reaction, and 3) unvaccinated elderly high-risk groups.
Methods
Based on the estimated target risk group population, we used a model of COVID-19 transmission to estimate the size of the risk group population for whom Evusheld treatment may help prevent symptomatic COVID-19 (and deaths) in 2022. We projected Evusheld intervention costs, quality-adjusted life year (QALY) lost, cost averted and QALY gained by reduced COVID-19 incidence, and incremental cost-effectiveness (cost per QALY gained) in each modeled population from the healthcare system perspective.
Results
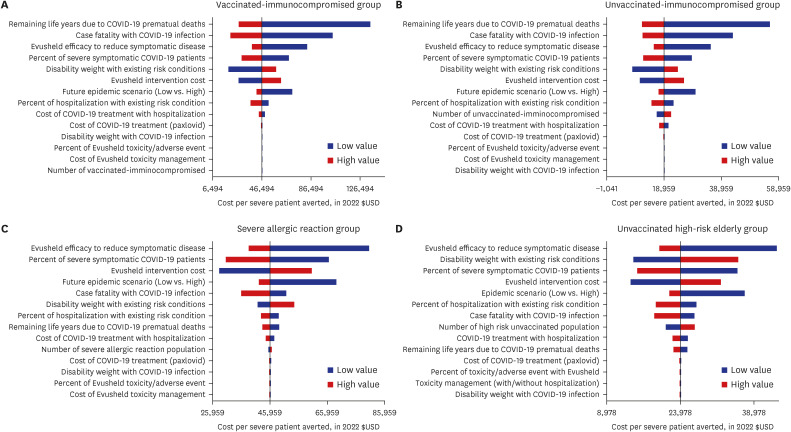
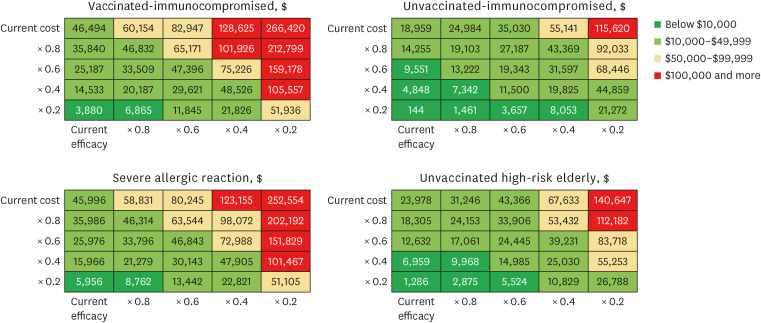
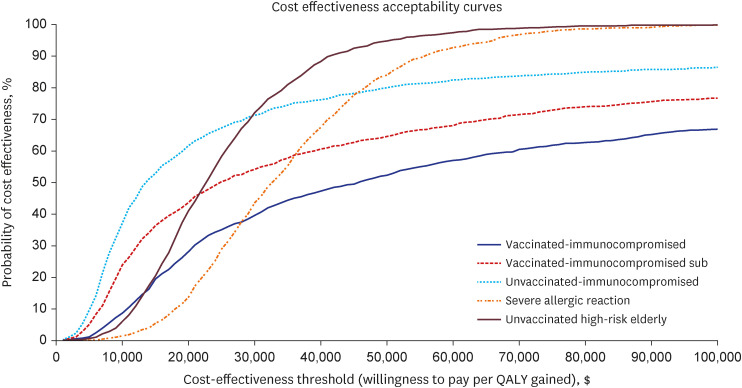
Our study demonstrated that Evusheld treatment for COVID-19 infection in South Korea is highly cost-effective for unvaccinated risk groups ($18,959 per QALY gained for immunocompromised and $23,978 per QALY gained for high-risk elderly groups) and moderately cost-effective among individuals who are vaccinated immunocompromised ($46,494 per QALY gained), or have severe allergic reactions ($45,996 per QALY gained). Evusheld’s cost-effectiveness may be subject to risk-group-specific COVID-19 disease progression and Evusheld efficacy and cost, which may change in future epidemic scenarios.
Conclusion
As the COVID-19 variants and risk group-specific durable efficacy, toxicity (and/ or resistance) and optimal dosing of Evusheld remain uncertain, better empirical estimates to inform these values in different epidemiological contexts are needed. These results may help decision-makers prioritize resources toward more equitable and effective COVID-19 control efforts.
Keyword
Figure
Reference
-
1. Kumari M, Lu RM, Li MC, Huang JL, Hsu FF, Ko SH, et al. A critical overview of current progress for COVID-19: development of vaccines, antiviral drugs, and therapeutic antibodies. J Biomed Sci. 2022; 29(1):68. PMID: 36096815.2. Khandia R, Singhal S, Alqahtani T, Kamal MA, El-Shall NA, Nainu F, et al. Emergence of SARS-CoV-2 omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ Res. 2022; 209:112816. PMID: 35093310.3. Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of COVID-19. N Engl J Med. 2022; 386(23):2188–2200. PMID: 35443106.4. Food and Drug Administration (US). FDA authorizes revisions to Evusheld dosing. Updated 2022. Accessed September 20, 2022. https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-revisions-evusheld-dosing .5. Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021; 385(21):1941–1950. PMID: 34706189.6. Food and Drug Administration (US). Fact sheet for healthcare providers: emergency use authorization of sotrovimab. Updated 2022. Accessed September 20, 2022. https://www.fda.gov/media/149534/download#:~:text=Sotrovimab%20is%20authorized%20for%20use,who%20are%20at%20high%20risk.2021 .7. Fred Hutchinson Cancer Center. Pharmacokinetics of sotrovimab as pre-exposure prophylaxis for COVID-19 in hematopoietic stem cell transplant recipients, COVIDMAB study. Updated 2023. Accessed March 1, 2023. https://clinicaltrials.gov/ct2/show/NCT05135650 .8. Totschnig D, Augustin M, Niculescu I, Laferl H, Jansen-Skoupy S, Lehmann C, et al. SARS-CoV-2 pre-exposure prophylaxis with sotrovimab and tixagevimab/cilgavimab in immunocompromised patients-a single-center experience. Viruses. 2022; 14(10):2278. PMID: 36298832.9. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med. 2021; 385(6):562–566. PMID: 34347959.10. Jo Y, Shrestha S, Radnaabaatar M, Park H, Jung J. Optimal social distancing policy for COVID-19 control in Korea: a model-based analysis. J Korean Med Sci. 2022; 37(23):e189. PMID: 35698839.11. Centers for Disease Control and Prevention (US). COVID-19 vaccines for people who are moderately or severely immunocompromised. Updated 2022. Accessed September 20, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html .12. National Institutes of Health (US). Prioritization of anti-SARS-CoV-2 Therapies for the treatment and prevention of COVID-19 when there are logistical or supply constraints. Updated 2022. Accessed September 20, 2022. https://www.covid19treatmentguidelines.nih.gov/overview/prioritization-of-therapeutics/ .13. Centers for Disease Control and Prevention (US). Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated 2022. Accessed September 20, 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html .14. Centers for Disease Control and Prevention (US). If you are having a severe allergic reaction to a COVID-19 vaccine. Updated 2022. Accessed September 20, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html .15. Statistics Korea. Population trends for March 2021. Updated 2021. Accessed September 20, 2022. https://kostat.go.kr/board.es?mid=a10301010000&bid=204&tag=&act=view&list_no=389818&ref_bid= .16. Health Insurance Review & Assessment Service (KR). Healthcare bigdata hub. Updated 2022. Accessed September 20, 2022. https://opendata.hira.or.kr/home.do .17. Korea Disease Control and Prevention Agency. Results of weekly analysis of adverse events after COVID-19 vaccination (week 62). Updated 2022. Accessed September 20, 2022. https://ncv.kdca.go.kr/board.es?mid=a11707010000&bid=0032&act=view&list_no=767&tag=&nPage=3 .18. Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006; 21(5):402–408. PMID: 16877455.19. Pereira MR, Mohan S, Cohen DJ, Husain SA, Dube GK, Ratner LE, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020; 20(7):1800–1808. PMID: 32330343.20. Washington State Department of Health. COVID-19 cases, hospitalizations, and deaths by vaccination status Washington State Department of Health. Published 2022. Accessed September 20, 2022. https://doh.wa.gov/emergencies/covid-19/data-dashboard#downloads .21. Pifarré I Arolas H, Acosta E, López-Casasnovas G, Lo A, Nicodemo C, Riffe T, et al. Author correction: years of life lost to COVID-19 in 81 countries. Sci Rep. 2021; 11(1):8543. PMID: 33854139.22. Parra PN, Atanasov V, Whittle J, Meurer J, Luo QE, Zhang R, et al. The effect of the COVID-19 pandemic on the elderly: population fatality rates, COVID mortality percentage, and life expectancy loss. Elder Law J. 2022; 30:33–80. PMID: 35936928.23. Andrasfay T, Goldman N. Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proc Natl Acad Sci U S A. 2021; 118(5):e2014746118. PMID: 33446511.24. Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022; 376:e069761. PMID: 35264324.25. Kim YE, Jo MW, Park H, Oh IH, Yoon SJ, Pyo J, et al. Updating disability weights for measurement of healthy life expectancy and disability-adjusted life year in Korea. J Korean Med Sci. 2020; 35(27):e219. PMID: 32657086.26. Reyes EA. This treatment can protect vulnerable people from COVID. But many don’t know about it. Updated 2022. Accessed September 20, 2022. https://www.latimes.com/california/story/2022-03-06/covid-antibody-treatment-obstacles .27. Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020; 395(10241):1919–1926. PMID: 32473682.28. Martín-Moro F, Marquet J, Piris M, Michael BM, Sáez AJ, Corona M, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020; 190(1):e16–e20. PMID: 32379921.29. Chanda D, Minchella PA, Kampamba D, Itoh M, Hines JZ, Fwoloshi S, et al. COVID-19 severity and COVID-19-associated deaths among hospitalized patients with HIV infection - Zambia, March-December 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(22):807–810. PMID: 34081684.30. National Institutes of Health (US). Prevention of SARS-CoV-2 infection. Updated 2022. Accessed September 20, 2022. https://www.covid19treatmentguidelines.nih.gov/overview/prevention-of-sars-cov-2/ .31. Mahase E. COVID-19: UK will not buy Evusheld owing to “insufficient data” on protection, government says. BMJ. 2022; 378:o2021. PMID: 35970520.32. Food and Drug Administration (US). FDA announces Evusheld is not currently authorized for emergency use in the U.S. Updated 2023. Accessed March 10, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us .33. VanBlargan LA, Errico JM, Halfmann PJ, Zost SJ, Crowe JE Jr, Purcell LA, et al. An infectious SARS-CoV-2 B.1.1.529 omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022; 28(3):490–495. PMID: 35046573.34. Park YJ, De Marco A, Starr TN, Liu Z, Pinto D, Walls AC, et al. Antibody-mediated broad sarbecovirus neutralization through ACE2 molecular mimicry. Science. 2022; 375(6579):449–454. PMID: 34990214.35. Food and Drug Administration (US). Letter of authorization for emergency use of REGEN-CoV reissue. Updated 2022. Accessed February 11, 2022. https://www.fda.gov/media/145610/download .36. Dijk SW, Krijkamp EM, Kunst N, Gross CP, Wong JB, Hunink MG. Emerging therapies for COVID-19: the value of information from more clinical trials. Value Health. 2022; 25(8):1268–1280. PMID: 35490085.37. Jovanoski N, Kuznik A, Becker U, Hussein M, Briggs A. Cost-effectiveness of casirivimab/imdevimab in patients with COVID-19 in the ambulatory setting. J Manag Care Spec Pharm. 2022; 28(5):555–565. PMID: 35238626.38. Flaxman AD, Issema R, Barnabas RV, Ross JM. Estimated health outcomes and costs of COVID-19 prophylaxis with monoclonal antibodies among unvaccinated household contacts in the US. JAMA Netw Open. 2022; 5(4):e228632. PMID: 35452104.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Loss of Neutralizing Activity of Tixagevimab/Cilgavimab (Evusheld™) Against Omicron BN.1, a Dominant Circulating Strain Following BA.5 During the Seventh Domestic Outbreak in Korea in Early 2023
- Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis
- HCV self-testing: Bridging screening gaps and ensuring cost-effectiveness for both high-risk and universal populations: Correspondence to editorial on “Self-testing strategy to eliminate hepatitis C as per World Health Organization’s goal: Analysis of disease burden and cost-effectiveness”
- Cost-effectiveness analysis of hospital treatment volume and survival outcomes in endometrial cancer in Japan
- RSV Prevention Strategies in Korean Children: A Review of Current Approaches and Emerging Options