Clin Transplant Res.
2024 Jun;38(2):136-144. 10.4285/ctr.24.0015.
Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Anesthesiology, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
- 2Department of Anesthesiology, School of Medicine, Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran
- 3Department of Anesthesiology, Paramedical Faculty, Hajar Hospital, AJA University of Medical Sciences, Tehran, Iran
- 4Department of Anesthesia and Critical Care, Sina Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 5School of Nursing, AJA University of Medical Sciences, Tehran, Iran
- KMID: 2557607
- DOI: http://doi.org/10.4285/ctr.24.0015
Abstract
- Background
Tixagevimab/cilgavimab (Tix/Cil) shows promise as a prophylactic treatment against coronavirus disease 2019 (COVID-19) in solid organ transplant recipients (SOTRs). This study was performed to assess the effectiveness of Tix/Cil for preexposure prophylaxis against COVID-19 in this population.
Methods
We systematically searched the Cochrane Library, Web of Science, PubMed, and Embase databases to identify articles relevant to our study up to December 15, 2023. Comprehensive Meta-Analysis (ver. 3.0) was used for data analysis.
Results
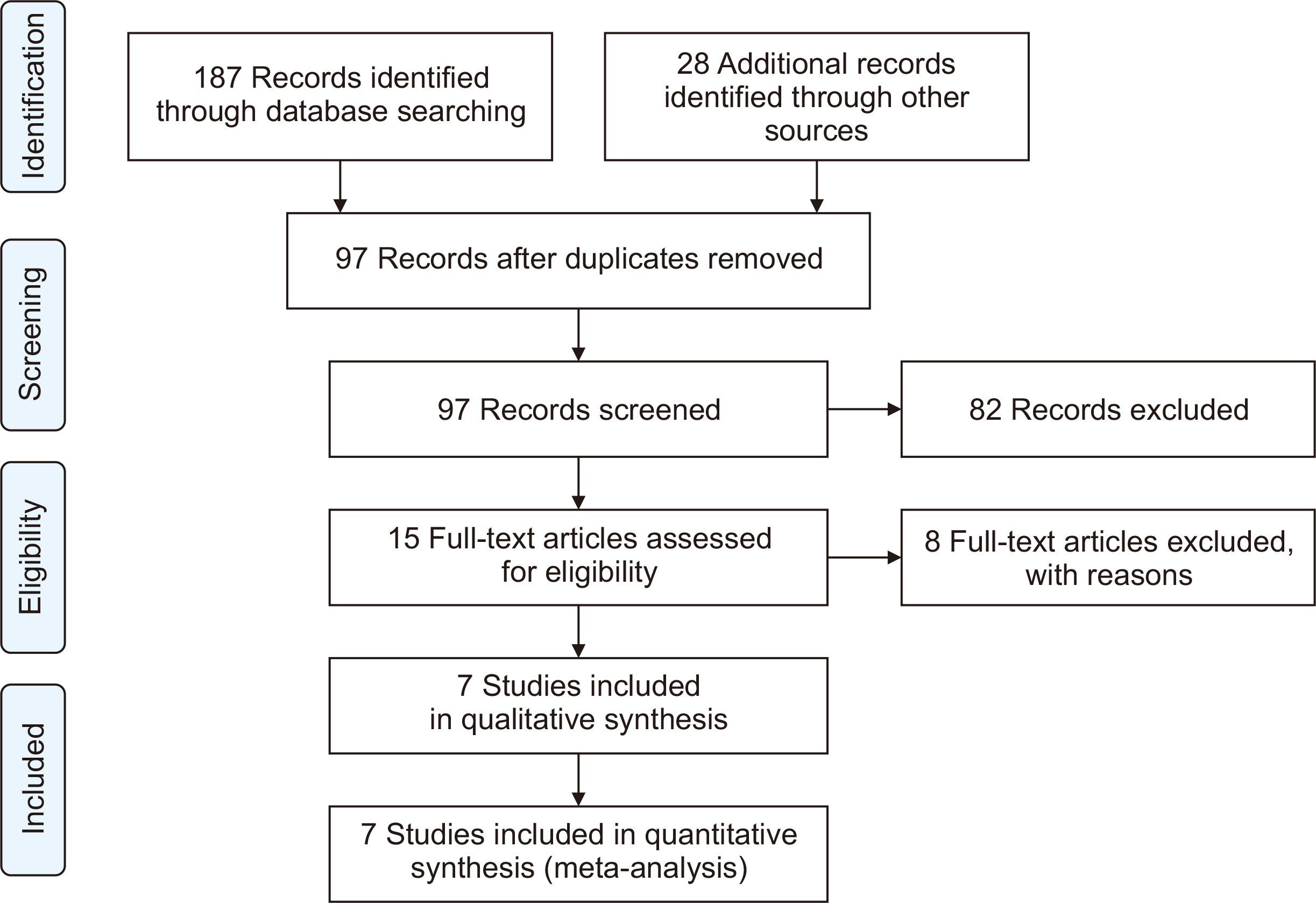
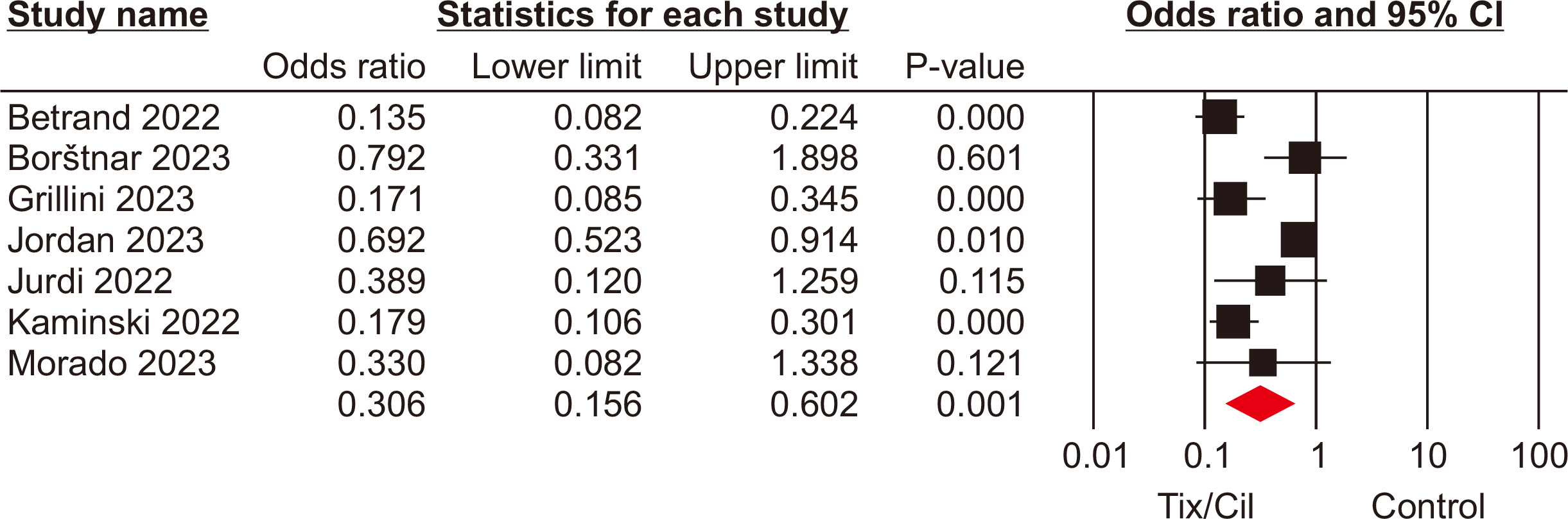
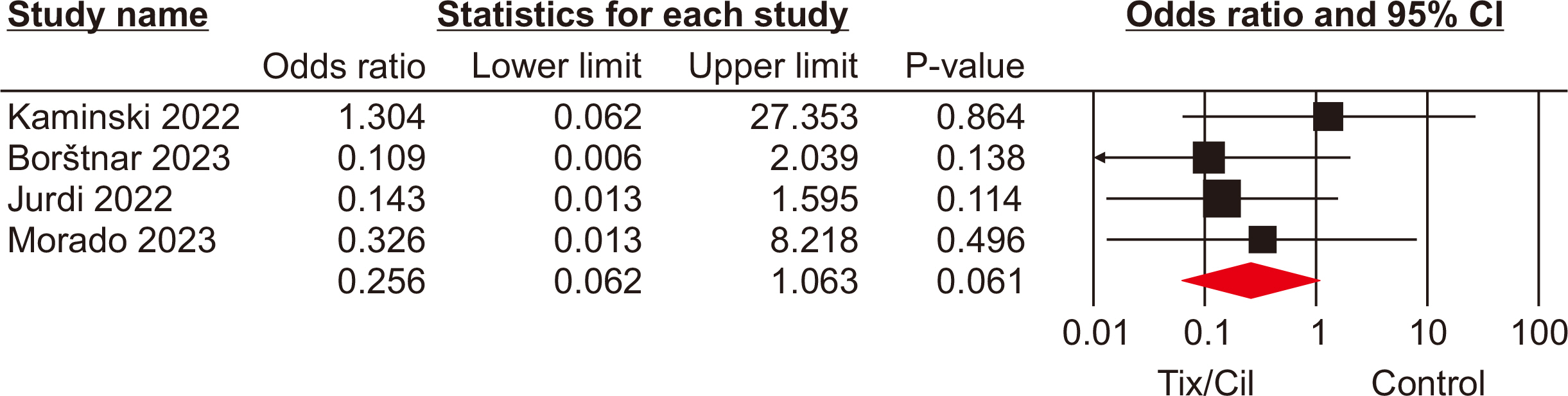
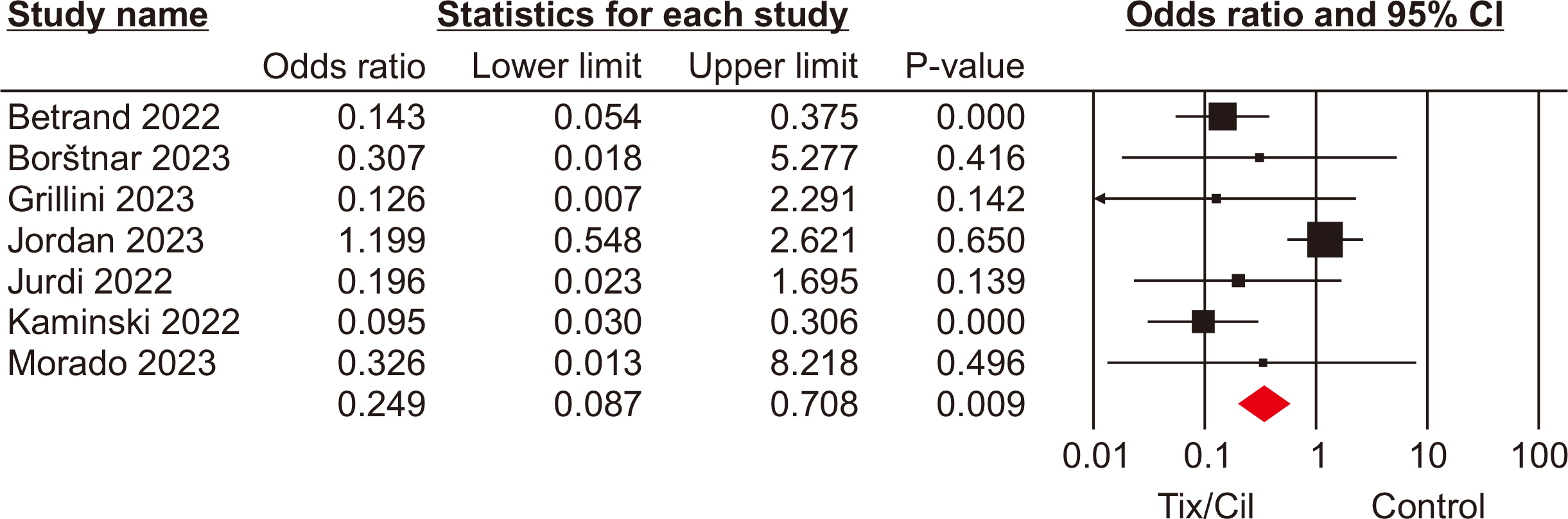
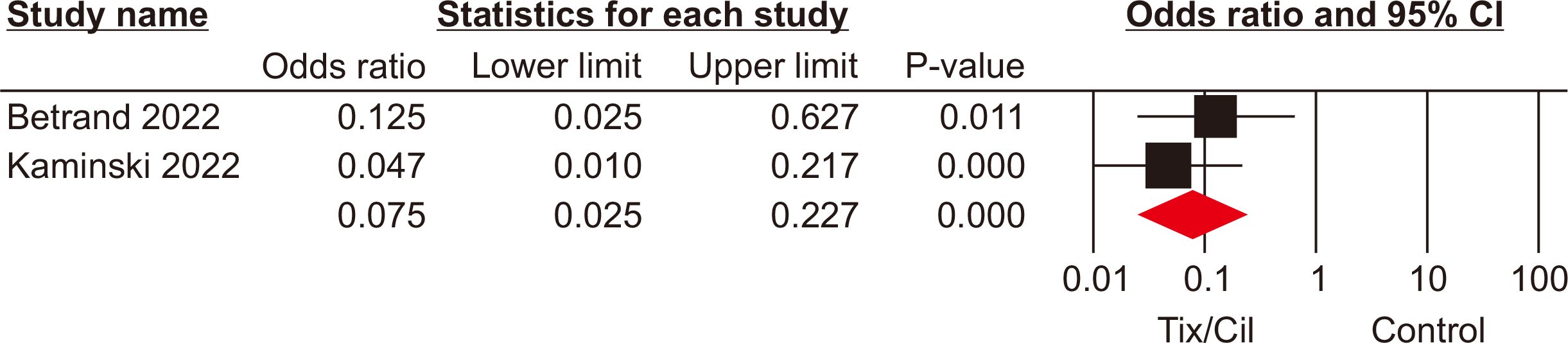
The meta-analysis included seven eligible retrospective studies, encompassing a total of 4,026 SOTRs. The analysis revealed significant differences in SOTRs who received Tix/Cil preexposure prophylaxis relative to those who did not. Specifically, these differences were observed in the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (odds ratio [OR], 0.30; 95% confidence interval [CI], 0.15–0.60), hospitalization (OR, 0.24; 95% CI, 0.08–0.70), and intensive care unit admission (OR, 0.07; 95% CI, 0.02–0.22). However, mortality rate did not differ significantly between the two groups (P=0.06).
Conclusions
The evidence supporting the effectiveness of Tix/Cil as preexposure prophylaxis against SARS-CoV-2 in SOTRs is of a low to moderate level. Further high-quality research is necessary to understand its effects on this population.
Keyword
Figure
Reference
-
1. Clarke JA, Wiemken TL, Korenblat KM. 2022; Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic. Transplantation. 106:2399–407. DOI: 10.1097/TP.0000000000004341. PMID: 36042551. PMCID: PMC9696767.2. Medina-Pestana J, Cristelli MP, Foresto RD, Tedesco-Silva H, Requião-Moura LR. 2022; The higher COVID-19 fatality rate among kidney transplant recipients calls for further action. Transplantation. 106:908–10. DOI: 10.1097/TP.0000000000004086. PMID: 35250005. PMCID: PMC9038247.
Article3. Peghin M, Graziano E, Grossi PA. 2022; SARS-CoV-2 vaccination in solid-organ transplant recipients. Vaccines (Basel). 10:1430. DOI: 10.3390/vaccines10091430. PMID: 36146506. PMCID: PMC9503203.
Article4. Yu B, Tamargo C, Brennan DC, Kant S. 2023; Measures to increase immunogenicity of SARS-CoV-2 vaccines in solid organ transplant recipients: a narrative review. Vaccines (Basel). 11:1755. DOI: 10.3390/vaccines11121755. PMID: 38140160. PMCID: PMC10748337.
Article5. Ryu TH, Kim HY, Ahn J, Oh JS, Kim JK. 2023; Delayed exacerbation of COVID-19 pneumonia in vaccinated kidney transplant recipients receiving immunosuppressants: a case series. Korean J Transplant. 37:63–8. DOI: 10.4285/kjt.22.0043. PMID: 37064773. PMCID: PMC10090830.
Article6. Amani B, Zareei S, Amani B, Zareei M, Zareei N, Shabestan R, et al. 2022; Artesunate, imatinib, and infliximab in COVID-19: a rapid review and meta-analysis of current evidence. Immun Inflamm Dis. 10:e628. DOI: 10.1002/iid3.628. PMID: 35634954. PMCID: PMC9092000.7. Amani B, Akbarzadeh A, Amani B, Shabestan R, Khorramnia S, Navidi Z, et al. 2023; Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis. J Med Virol. 95:e28889. DOI: 10.1002/jmv.28889. PMID: 37368841.8. Yang M, Li T, Wang Y, Tran C, Zhao S, Ao G. 2022; Monoclonal antibody therapy improves severity and mortality of COVID-19 in organ transplant recipients: a meta-analysis. J Infect. 85:436–80. DOI: 10.1016/j.jinf.2022.06.027. PMCID: PMC9247112. PMID: 35788012.
Article9. Morado F, Davoudi R, Kawewat-Ho P, Nanda N, Cartus R, Shaikh SA. 2023; A single-center review of pre-exposure prophylaxis with tixagevimab-cilgavimab in solid organ transplant recipients. Transpl Infect Dis. 25:e14086. DOI: 10.1111/tid.14086. PMID: 37314092.10. Capoluongo N, Mascolo A, Bernardi FF, Sarno M, Mattera V, di Flumeri G, et al. 2023; Retrospective analysis of a real-life use of tixagevimab-cilgavimab plus SARS-CoV-2 antivirals for treatment of COVID-19. Pharmaceuticals (Basel). 16:1493. DOI: 10.3390/ph16101493. PMID: 37895964. PMCID: PMC10609705.11. Food and Drug Administration (FDA). 2023. FDA announces Evusheld is not currently authorized for emergency use in the U.S. [Internet]. FDA;Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us. cited 2023 Dec 2.12. Borštnar Š, Arnol M, Večerić Haler Ž, Mlinšek G. Preexposure prophylaxis against COVID-19 with tixagevimab/cilgavimab in Slovenian national cohort of kidney transplant recipients. Preprints.org [Preprint]. 2023. Available from: https://doi.org/10.20944/preprints202306.0122.v1. cited 2023 Oct 11. DOI: 10.20944/preprints202306.0122.v1.
Article13. Jordan SC, Joung SY, Wang M, Tran TA, Bravo M, Masoom H, et al. 2024; Assessing the post hoc effectiveness of tixagevimab-cilgavimab for prevention of SARS-CoV-2 infections in solid organ transplant recipients. Transpl Infect Dis. 26:e14182. DOI: 10.1111/tid.14182. PMID: 37885435. PMCID: PMC10922395.
Article14. Alhumaid S, Al Mutair A, Alali J, Al Dossary N, Albattat SH, Al HajjiMohammed SM, et al. 2022; Efficacy and safety of tixagevimab/cilgavimab to prevent COVID-19 (pre-exposure prophylaxis): a systematic review and meta-analysis. Diseases. 10:118. DOI: 10.3390/diseases10040118. PMID: 36547204. PMCID: PMC9777759.
Article15. Soeroto AY, Yanto TA, Kurniawan A, Hariyanto TI. 2023; Efficacy and safety of tixagevimab-cilgavimab as pre-exposure prophylaxis for COVID-19: a systematic review and meta-analysis. Rev Med Virol. 33:e2420. DOI: 10.1002/rmv.2420. PMID: 36617704.
Article16. Jurdi AA, Morena L, Cote M, Bethea E, Azzi J, Riella LV. 2022; Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the Omicron wave. Am J Transplant. 22:3130–6. DOI: 10.1111/ajt.17128. PMID: 35727916. PMCID: PMC9906353.
Article17. Bertrand D, Laurent C, Lemée V, Lebourg L, Hanoy M, Le Roy F, et al. 2022; Efficacy of anti-SARS-CoV-2 monoclonal antibody prophylaxis and vaccination on the Omicron variant of COVID-19 in kidney transplant recipients. Kidney Int. 102:440–2. DOI: 10.1016/j.kint.2022.05.007. PMID: 35618097. PMCID: PMC9125992.
Article18. Kaminski H, Gigan M, Vermorel A, Charrier M, Guirle L, Jambon F, et al. 2022; COVID-19 morbidity decreases with tixagevimab-cilgavimab preexposure prophylaxis in kidney transplant recipient nonresponders or low-vaccine responders. Kidney Int. 102:936–8. DOI: 10.1016/j.kint.2022.07.008. PMID: 35870641. PMCID: PMC9297679.
Article19. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. 2015; Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 4:1. DOI: 10.1186/2046-4053-4-1. PMID: 25554246. PMCID: PMC4320440.
Article20. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.
Article21. Grillini A, Stracener P, Scarola D, Lyons J, Dilling D. 2023; Use of tixagevimab and cilgavimab (Evusheld) and subsequent outcomes of SARS-CoV-2 infections in lung transplant recipients. J Heart Lung Transplant. 42(4 Suppl):S311. DOI: 10.1016/j.healun.2023.02.715. PMCID: PMC10068082.22. Chen X, Luo D, Mei B, Du J, Liu X, Xie H, et al. 2023; Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and meta-analysis. Clin Microbiol Infect. 29:441–56. DOI: 10.1016/j.cmi.2022.12.004. PMID: 36509376. PMCID: PMC9733302.
Article23. Ouyang J, Zaongo SD, Harypursat V, Li X, Routy JP, Chen Y. 2022; SARS-CoV-2 pre-exposure prophylaxis: a potential COVID-19 preventive strategy for high-risk populations, including healthcare workers, immunodeficient individuals, and poor vaccine responders. Front Public Health. 10:945448. DOI: 10.3389/fpubh.2022.945448. PMID: 36003629. PMCID: PMC9393547.
Article24. Sun J, Zheng Q, Madhira V, Olex AL, Anzalone AJ, Vinson A, et al. 2022; Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 182:153–62. DOI: 10.1001/jamainternmed.2021.7024. PMID: 34962505. PMCID: PMC8715386.
Article25. O'Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. 2021; Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 385:1184–95. DOI: 10.1056/NEJMoa2109682. PMID: 34347950. PMCID: PMC8362593.26. Cohen MS, Nirula A, Mulligan MJ, Novak RM, Marovich M, Yen C, et al. 2021; Effect of bamlanivimab vs placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial. JAMA. 326:46–55. DOI: 10.1001/jama.2021.8828. PMID: 34081073. PMCID: PMC8176388.
Article27. Amani B, Shabestan R, Rajabkhah K, Amani B. 2023; Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis. Korean J Transplant. 37:277–85. DOI: 10.4285/kjt.23.0038. PMID: 37916433. PMCID: PMC10772269.
Article28. Case JB, Mackin S, Errico JM, Chong Z, Madden EA, Whitener B, et al. 2022; Resilience of S309 and AZD7442 monoclonal antibody treatments against infection by SARS-CoV-2 Omicron lineage strains. Nat Commun. 13:3824. DOI: 10.1038/s41467-022-31615-7. PMID: 35780162. PMCID: PMC9250508.29. Focosi D, Casadevall A. 2022; A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (EvusheldTM) for COVID-19 prophylaxis and treatment. Viruses. 14:1999. DOI: 10.3390/v14091999. PMID: 36146805. PMCID: PMC9505619.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The first case of brain death organ donation in a positive COVID-19 donors in Korea
- Remdesivir in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
- Loss of Neutralizing Activity of Tixagevimab/Cilgavimab (Evusheld™) Against Omicron BN.1, a Dominant Circulating Strain Following BA.5 During the Seventh Domestic Outbreak in Korea in Early 2023
- Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
- SARS-CoV-2 vaccine-elicited immune responses in solid organ transplant recipients