Clin Transplant Res.
2024 Sep;38(3):212-221. 10.4285/ctr.24.0031.
Remdesivir in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Anesthesiology, School of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
- 2Department of Anesthesiology, Faculty of Paramedical Sciences, AJA University of Medical Sciences, Tehran, Iran
- 3Department of Anesthesiology, Imam Khomeini Hospital Complex, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Anesthesia and Critical Care, Sina Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 5Department of Intensive Care Medicine, Imam Khomeini Hospital Complex, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 6Department of Orthopedics, School of Medicine, AJA University of Medical Sciences, Tehran, Iran
- 7School of Nursing, AJA University of Medical Sciences, Tehran, Iran
- KMID: 2559810
- DOI: http://doi.org/10.4285/ctr.24.0031
Abstract
- Background
The use of remdesivir in solid organ transplant recipients (SOTRs) with coronavirus disease 2019 (COVID-19) has been studied. The present systematic review and analysis aimed to assess its effectiveness in this population.
Methods
A comprehensive search of PubMed, Cochrane Library, Web of Science, Embase, medRxiv, and Google Scholar was conducted to identify relevant articles published up to April 2024. The quality of the included studies was evaluated using the Cochrane assessment tool. Data analysis was performed using the Comprehensive Meta-Analysis software ver. 3.0.
Results
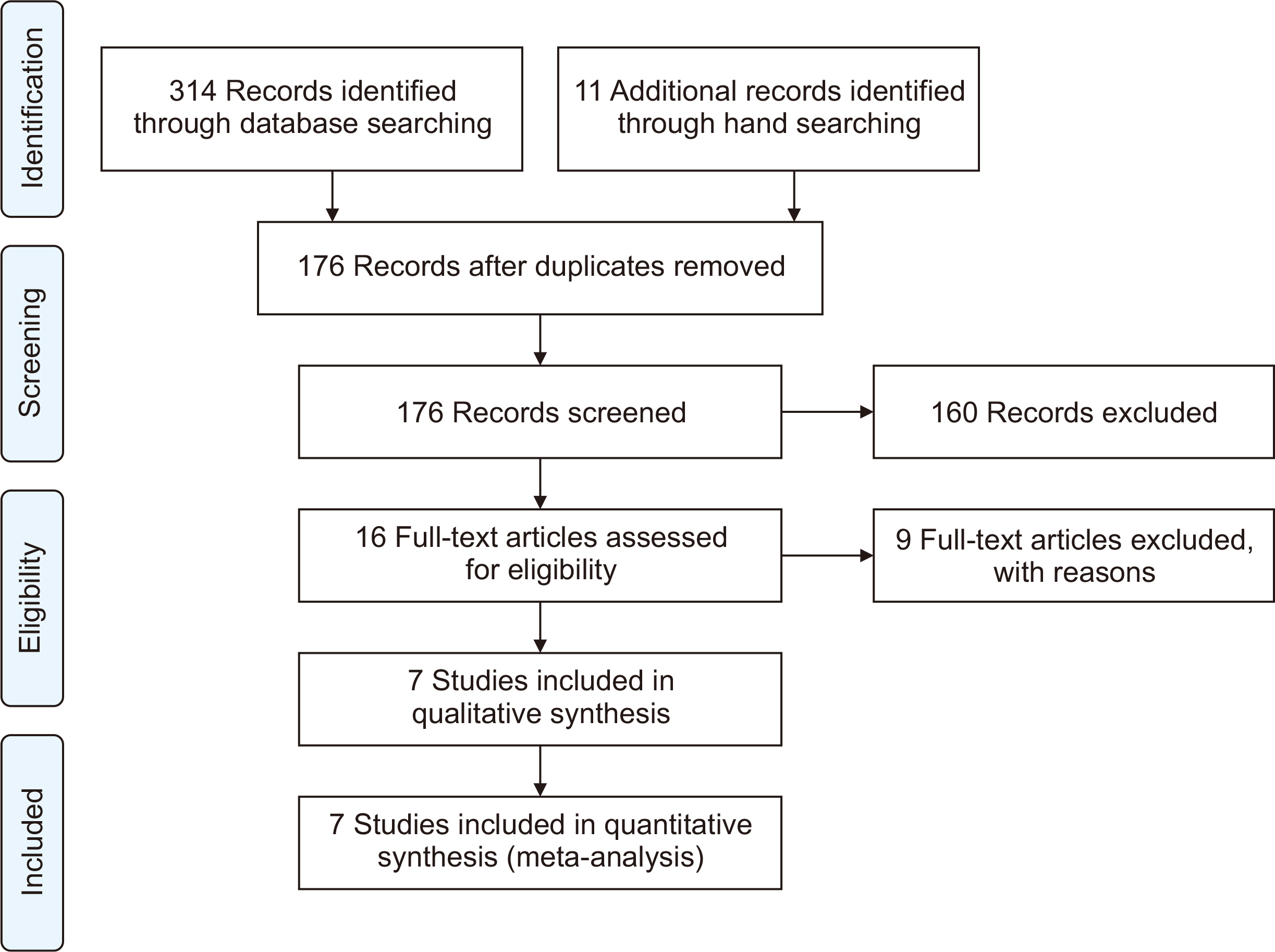
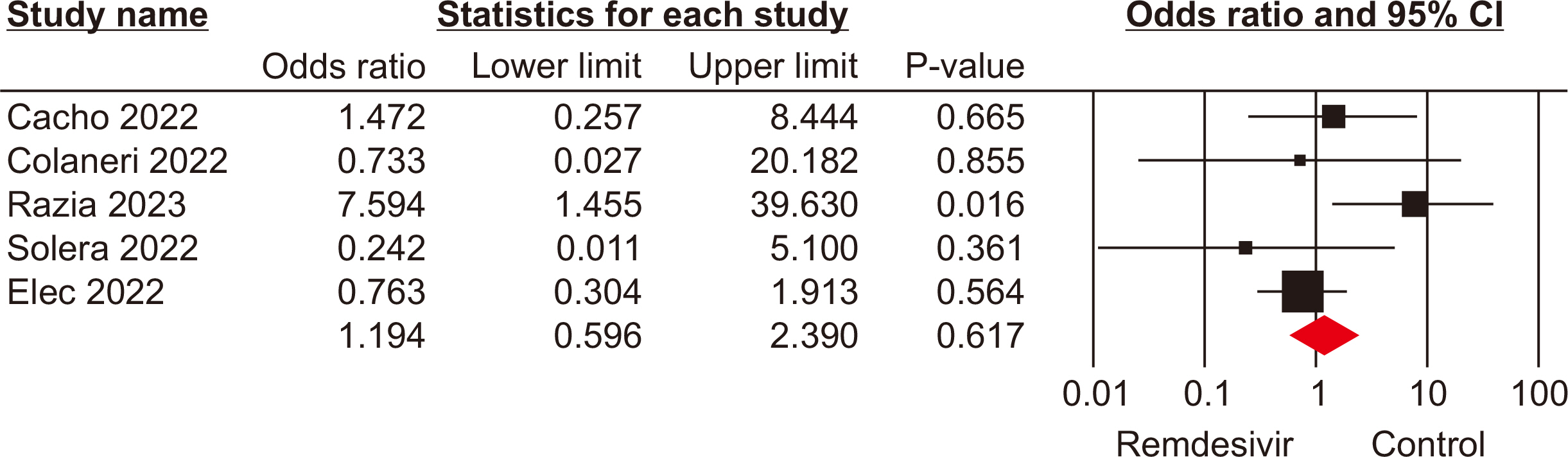
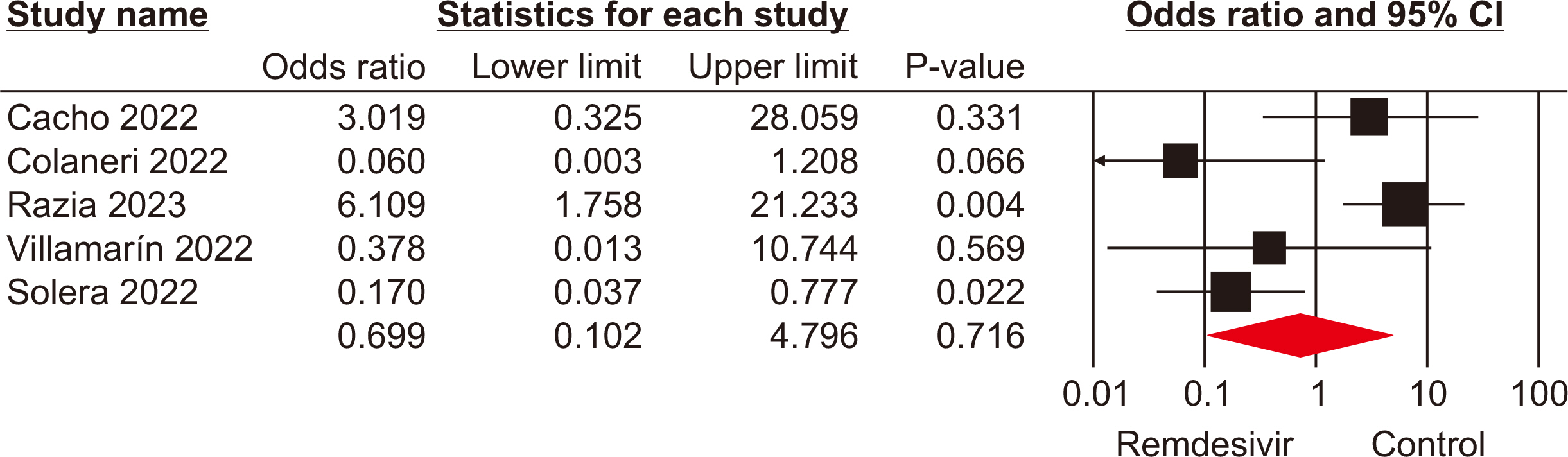
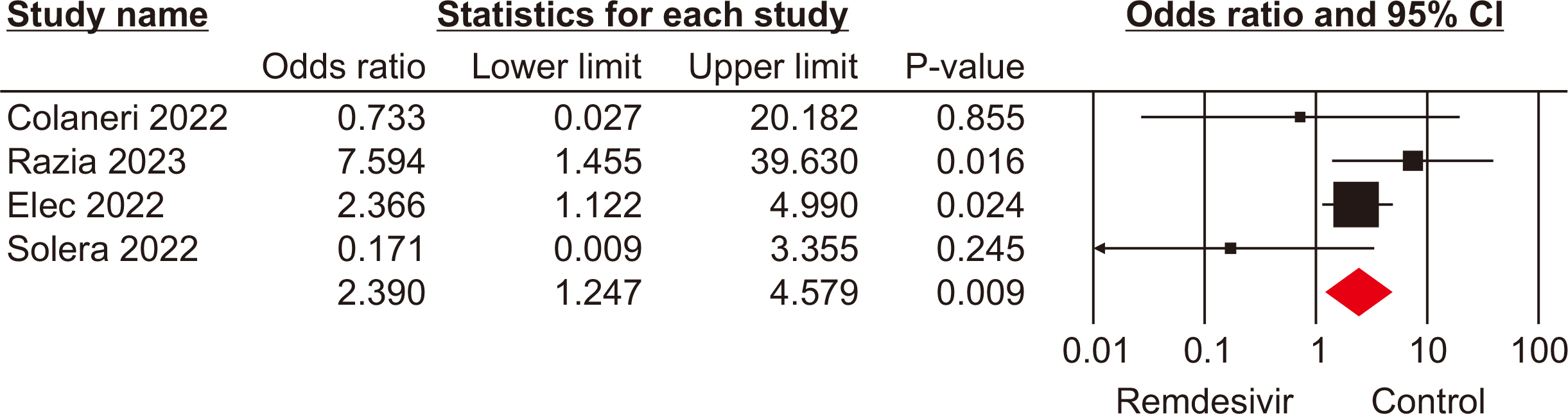
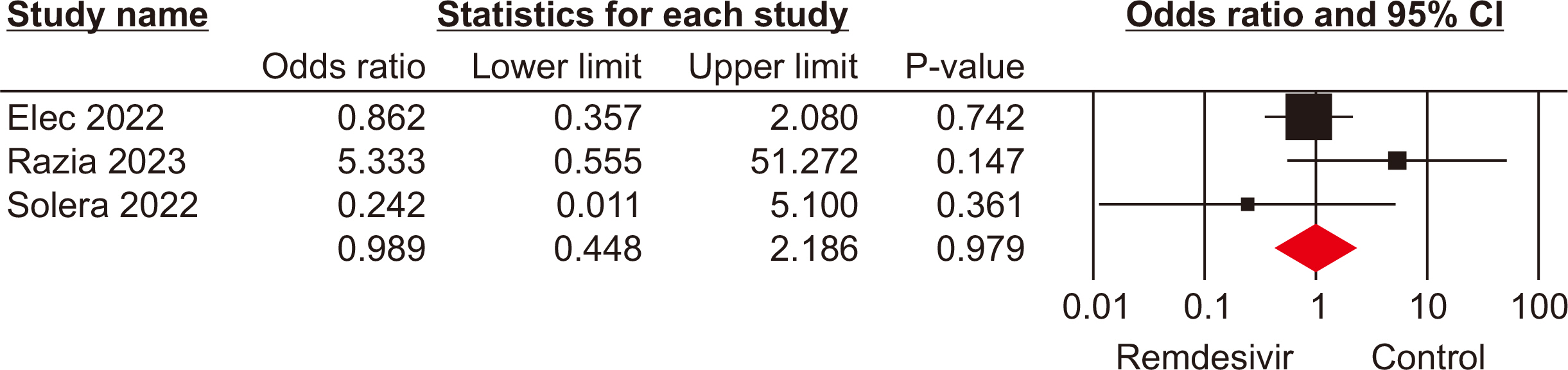
The meta-analysis included seven eligible retrospective studies, involving a total of 574 SOTRs. The findings indicated no significant differences in mortality rate (odds ratio [OR], 1.19; 95% confidence interval [CI], 0.59–2.39), hospitalization rate (OR, 0.69; 95% CI, 0.10–4.79), need for mechanical ventilation (OR, 0.98; 95% CI, 0.44–2.18), or need for oxygen therapy (OR, 3.73; 95% CI, 0.75–18.34) between the groups that received remdesivir and those that did not. However, a statistically significant difference was observed in the rate of intensive care unit admissions between the two groups (OR, 2.39; 95% CI, 1.24–4.57).
Conclusions
Our meta-analysis found that remdesivir offers no clinical benefits to SOTRs infected with COVID-19. Additional high-quality research is required to assess the potential clinical advantages of remdesivir for SOTRs with COVID-19.
Keyword
Figure
Reference
-
1. Danziger-Isakov L, Blumberg EA, Manuel O, Sester M. 2021; Impact of COVID-19 in solid organ transplant recipients. Am J Transplant. 21:925–37. DOI: 10.1111/ajt.16449. PMID: 33319449. PMCID: PMC9800718.2. Clarke JA, Wiemken TL, Korenblat KM. 2022; Excess mortality among solid organ transplant recipients in the United States during the COVID-19 pandemic. Transplantation. 106:2399–407. DOI: 10.1097/TP.0000000000004341. PMID: 36042551. PMCID: PMC9696767.3. Søfteland JM, Li H, Magnusson JM, Leach S, Friman V, Gisslén M, et al. 2024; COVID-19 outcomes and vaccinations in Swedish solid organ transplant recipients 2020-2021: a nationwide multi-register comparative cohort study. Viruses. 16:271. DOI: 10.3390/v16020271. PMID: 38400046. PMCID: PMC10893154.4. Ryu TH, Kim HY, Ahn J, Oh JS, Kim JK. 2023; Delayed exacerbation of COVID-19 pneumonia in vaccinated kidney transplant recipients receiving immunosuppressants: a case series. Korean J Transplant. 37:63–8. DOI: 10.4285/kjt.22.0043. PMID: 37064773. PMCID: PMC10090830.5. Mahalingasivam V, Craik A, Tomlinson LA, Ge L, Hou L, Wang Q, et al. 2021; A systematic review of COVID-19 and kidney transplantation. Kidney Int Rep. 6:24–45. DOI: 10.1016/j.ekir.2020.10.023. PMID: 33163708. PMCID: PMC7607258.6. Jering KS, McGrath MM, Mc Causland FR, Claggett B, Cunningham JW, Solomon SD. 2022; Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. 36:e14492. DOI: 10.1111/ctr.14492. PMID: 34558116. PMCID: PMC8646895.7. Villanego F, Vigara LA, Alonso M, Orellana C, Gómez AM, Eady M, et al. 2022; Trends in COVID-19 outcomes in kidney transplant recipients during the period of omicron variant predominance. Transplantation. 106:e304–5. DOI: 10.1097/TP.0000000000004126. PMID: 35389374. PMCID: PMC9128401.8. Amani B, Zareei S, Amani B, Zareei M, Zareei N, Shabestan R, et al. 2022; Artesunate, imatinib, and infliximab in COVID-19: a rapid review and meta-analysis of current evidence. Immun Inflamm Dis. 10:e628. DOI: 10.1002/iid3.628. PMID: 35634954. PMCID: PMC9092000.9. Chen X, Luo D, Mei B, Du J, Liu X, Xie H, et al. 2023; Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: a systematic review and meta-analysis. Clin Microbiol Infect. 29:441–56. DOI: 10.1016/j.cmi.2022.12.004. PMID: 36509376. PMCID: PMC9733302.10. Huh K, Kang M, Kim YE, Choi Y, An SJ, Seong J, et al. 2024; Risk of severe COVID-19 and protective effectiveness of vaccination among solid organ transplant recipients. J Infect Dis. 229:1026–34. DOI: 10.1093/infdis/jiad501. PMID: 38097377.11. Gao Y, Liu M, Li Z, Xu J, Zhang J, Tian J. 2023; Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin Microbiol Infect. 29:979–99. DOI: 10.1016/j.cmi.2023.04.014. PMID: 37084941. PMCID: PMC10116122.12. Amani B, Amani B. 2024; Comparison of effectiveness and safety of nirmatrelvir/ritonavir versus sotrovimab for COVID-19: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 1–9. DOI: 10.1080/14787210.2024.2326561. PMID: 38457124.13. Amani B, Akbarzadeh A, Amani B, Shabestan R, Khorramnia S, Navidi Z, et al. 2023; Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis. J Med Virol. 95:e28889. DOI: 10.1002/jmv.28889. PMID: 37368841.14. Angamo MT, Mohammed MA, Peterson GM. 2022; Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis. Infection. 50:27–41. DOI: 10.1007/s15010-021-01671-0. PMID: 34331674. PMCID: PMC8325414.15. Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. 2020; Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. 585:273–6. DOI: 10.1038/s41586-020-2423-5. PMID: 32516797. PMCID: PMC7486271.16. Cacho J, Nicolás D, Bodro M, Cuadrado-Payán E, Torres-Jaramillo V, Gonzalez-Rojas Á, et al. 2022; Use of remdesivir in kidney transplant recipients with SARS-CoV-2 Omicron infection. Kidney Int. 102:917–21. DOI: 10.1016/j.kint.2022.08.001. PMID: 35964801. PMCID: PMC9367173.17. Fesu D, Bohacs A, Hidvegi E, Matics Z, Polivka L, Horvath P, et al. 2022; Remdesivir in solid organ recipients for COVID-19 pneumonia. Transplant Proc. 54:2567–9. DOI: 10.1016/j.transproceed.2022.10.043. PMID: 36400587. PMCID: PMC9626440.18. Solera JT, Árbol BG, Bahinskaya I, Marks N, Humar A, Kumar D. 2023; Short-course early outpatient remdesivir prevents severe disease due to COVID-19 in organ transplant recipients during the omicron BA.2 wave. Am J Transplant. 23:78–83. DOI: 10.1111/ajt.17199. PMID: 36148607. PMCID: PMC9538117.19. Villamarín M, Márquez-Algaba E, Esperalba J, Perelló M, Los Arcos I, Company D, et al. 2022; Preliminary clinical experience of molnupiravir to prevent progression of COVID-19 in kidney transplant recipients. Transplantation. 106:2200–4. DOI: 10.1097/TP.0000000000004306. PMID: 35915545.20. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. 2009; Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 151:264–9. DOI: 10.7326/0003-4819-151-4-200908180-00135. PMID: 19622511.21. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.22. Zhang Y, Coello PA, Guyatt GH, Yepes-Nuñez JJ, Akl EA, Hazlewood G, et al. 2019; GRADE guidelines: 20. assessing the certainty of evidence in the importance of outcomes or values and preferences-inconsistency, imprecision, and other domains. J Clin Epidemiol. 111:83–93. DOI: 10.1016/j.jclinepi.2018.05.011. PMID: 29800687.23. Colaneri M, Amarasinghe N, Rezzonico L, Pieri TC, Segalini E, Sambo M, et al. 2022; Early remdesivir to prevent severe COVID-19 in recipients of solid organ transplant: a real-life study from Northern Italy. Int J Infect Dis. 121:157–60. DOI: 10.1016/j.ijid.2022.05.001. PMID: 35533831. PMCID: PMC9076039.24. Elec F, Magnusson J, Elec A, Muntean A, Antal O, Moisoiu T, et al. 2022; COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome - a retrospective cohort study. Int J Infect Dis. 118:247–53. DOI: 10.1016/j.ijid.2022.03.015. PMID: 35301103. PMCID: PMC8920078.25. Razia D, Sindu D, Grief K, Cherrier L, Omar A, Walia R, et al. 2023; Molnupiravir vs remdesivir for treatment of Covid-19 in lung transplant recipients. J Heart Lung Transplant. 42:S165–6. DOI: 10.1016/j.healun.2023.02.1653.26. Khorramnia S, Navidi Z, Orandi A, Iravani MM, Orandi A, Malekabad ES, et al. 2024; Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis. Clin Transplant Res. 38:136–44. DOI: 10.4285/ctr.24.0015. PMID: 38904088. PMCID: PMC11228381.27. Wong CK, Lau KT, Au IC, Xiong X, Chung MS, Lau EH, et al. 2022; Optimal timing of remdesivir initiation in hospitalized patients with coronavirus disease 2019 (COVID-19) administered with dexamethasone. Clin Infect Dis. 75:e499–508. DOI: 10.1093/cid/ciab728. PMID: 34420051. PMCID: PMC8513400.28. Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. 2021; Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. 31:e2187. DOI: 10.1002/rmv.2187. PMID: 33128490.29. Amani B, Shabestan R, Rajabkhah K, Amani B. 2023; Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis. Korean J Transplant. 37:277–85. DOI: 10.4285/kjt.23.0038. PMID: 37916433. PMCID: PMC10772269.30. Dhand A, Okumura K, Ohira S, Kapur R, Wolfe K, Nishida S. 2023; Molnupiravir for treatment of COVID-19 in solid organ transplant recipients. Transplantation. 107:e182–3. DOI: 10.1097/TP.0000000000004588. PMID: 36959161. PMCID: PMC10205063.31. Tang Y, Li Y, Song T. 2023; Optimizing the use of nirmatrelvir/ritonavir in solid organ transplant recipients with COVID-19: a review of immunosuppressant adjustment strategies. Front Immunol. 14:1150341. DOI: 10.3389/fimmu.2023.1150341. PMID: 37081880. PMCID: PMC10111375.32. Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, et al. 2022; Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 386:305–15. DOI: 10.1056/NEJMoa2116846. PMID: 34937145. PMCID: PMC8757570.33. Mazzitelli M, Trunfio M, Sasset L, Scaglione V, Ferrari A, Mengato D, et al. 2023; Risk of hospitalization and sequelae in patients with COVID-19 treated with 3-day early remdesivir vs. controls in the vaccine and Omicron era: a real-life cohort study. J Med Virol. 95:e28660. DOI: 10.1002/jmv.28660. PMID: 36905216.34. Alonso-Navarro R, Ramírez M, Masiá M, Paredes R, Montejano R, Povar-Echeverria M, et al. 2023; Time from symptoms onset to remdesivir is associated with the risk of ICU admission: a multicentric analyses. BMC Infect Dis. 23:286. DOI: 10.1186/s12879-023-08222-y. PMID: 37142994. PMCID: PMC10157565.35. Reddy Vegivinti CT, Pederson JM, Saravu K, Gupta N, Barrett A, Davis AR, et al. 2021; Remdesivir therapy in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond). 62:43–8. DOI: 10.1016/j.amsu.2020.12.051. PMID: 33489115. PMCID: PMC7806502.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumonia
- Systematic Review for Remdesivir Use in Pediatric Patients under 3.5 kg with COVID-19
- The first case of brain death organ donation in a positive COVID-19 donors in Korea
- Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
- Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis