Korean J Transplant.
2023 Dec;37(4):277-285. 10.4285/kjt.23.0038.
Sotrovimab in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
- Affiliations
-
- 1Department of Health Management and Economics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 2Department of Biostatistics and Epidemiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 3Department of Curative Affairs, Ministry of Health and Medical Education, Tehran, Iran
- KMID: 2550239
- DOI: http://doi.org/10.4285/kjt.23.0038
Abstract
- Background
Despite widespread implementation of vaccination against coronavirus disease 2019 (COVID-19), solid organ transplant recipients (SOTRs) can remain partic-ularly vulnerable to this disease. The present study was conducted to investigate the efficacy and safety of sotrovimab in the treatment of SOTRs with COVID-19.
Methods
A search was performed of PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar to gather relevant evidence through July 25, 2023. The quality of the included studies was assessed using the risk of bias tool. Comprehensive Meta-Analysis software (ver. 3.0, Biostat) was employed for data analysis.
Results
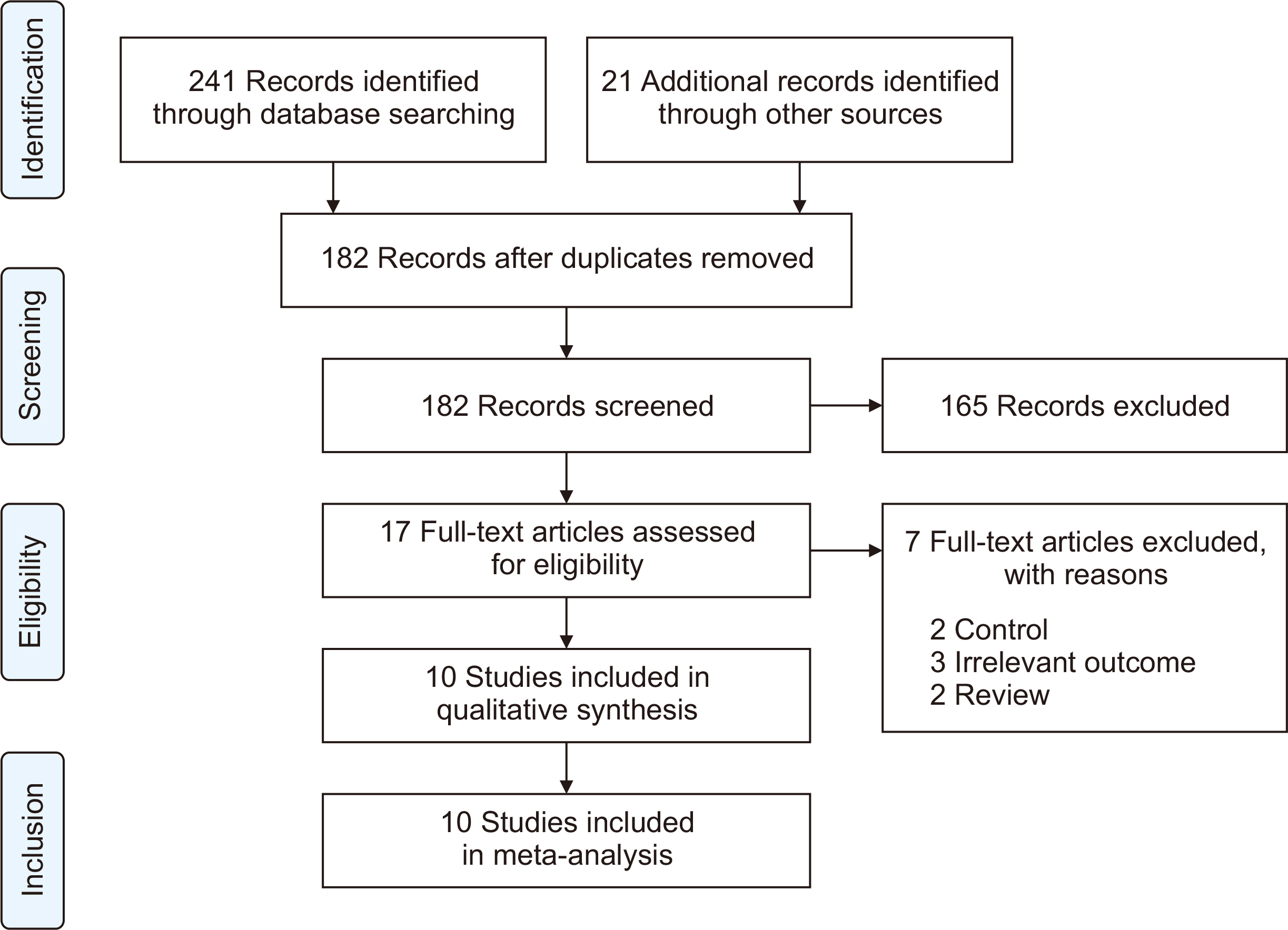
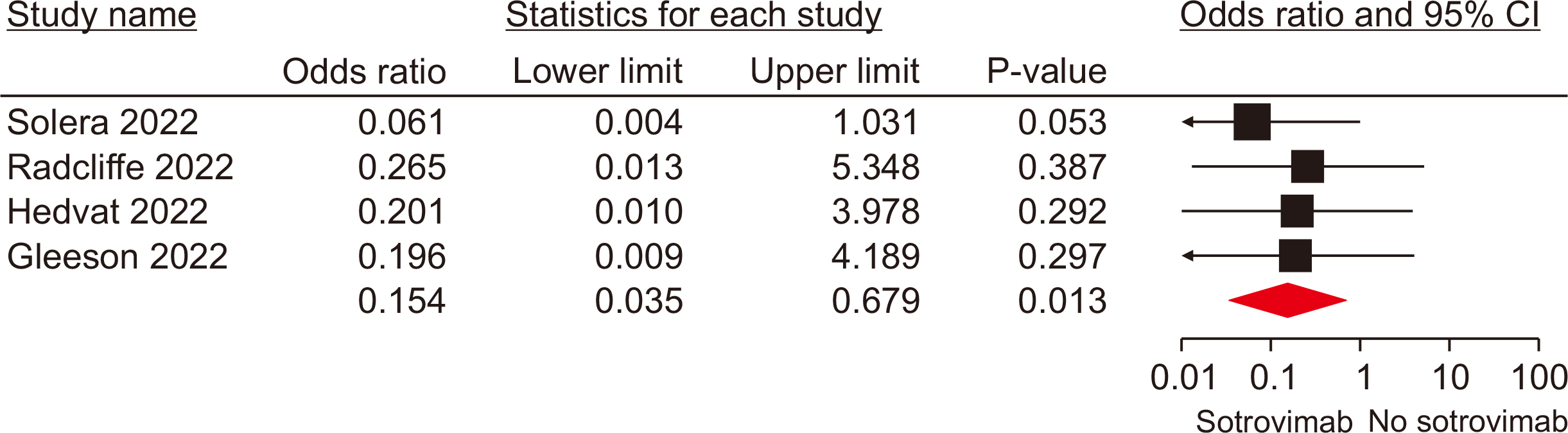
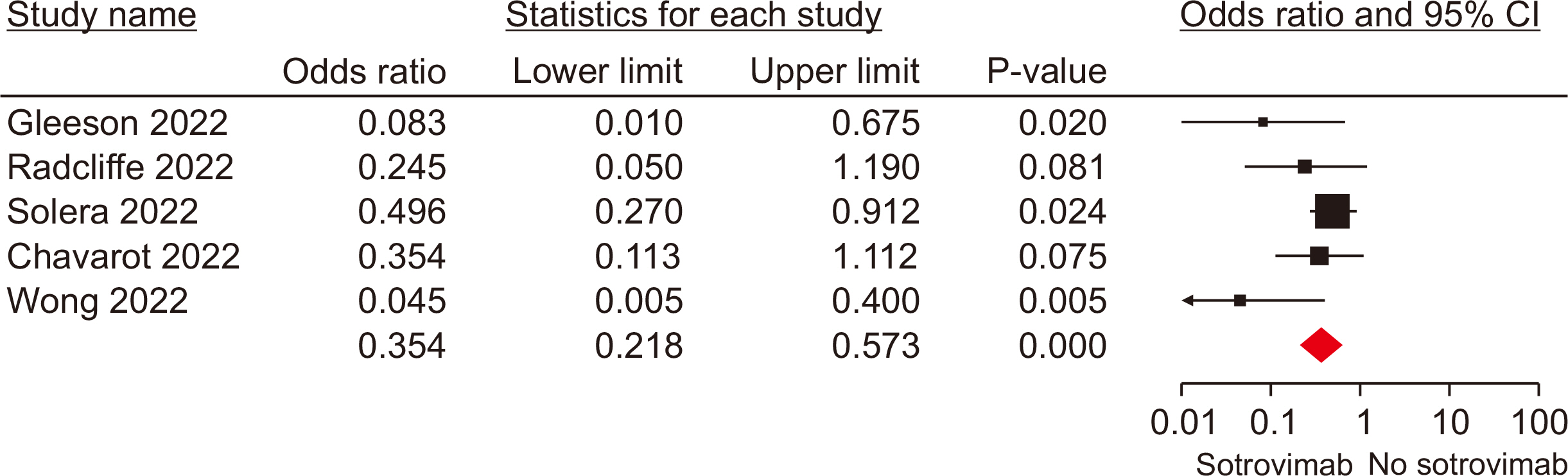
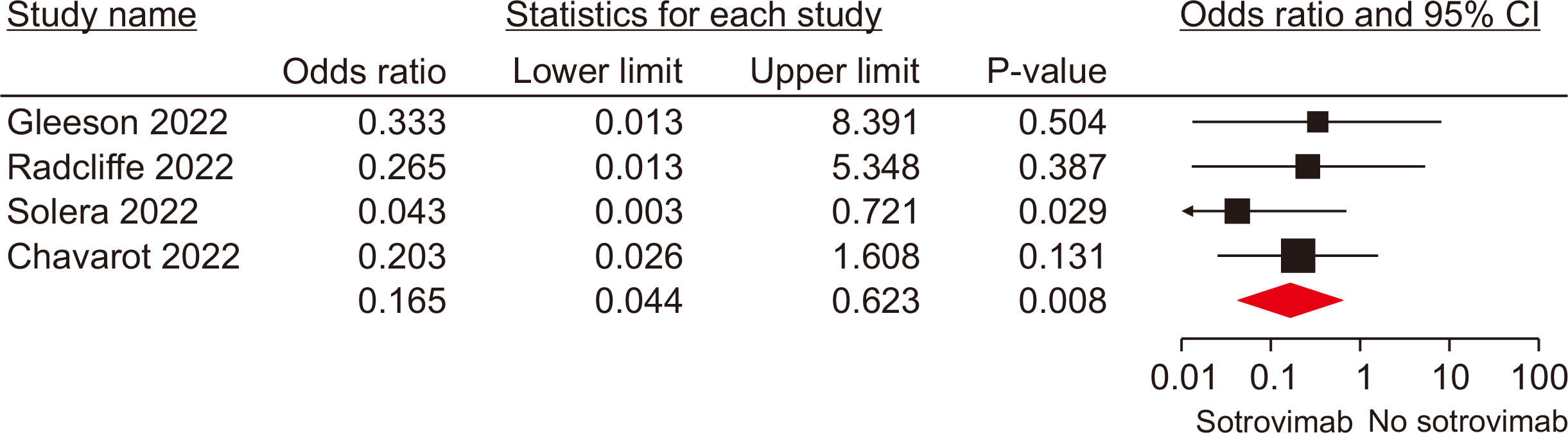
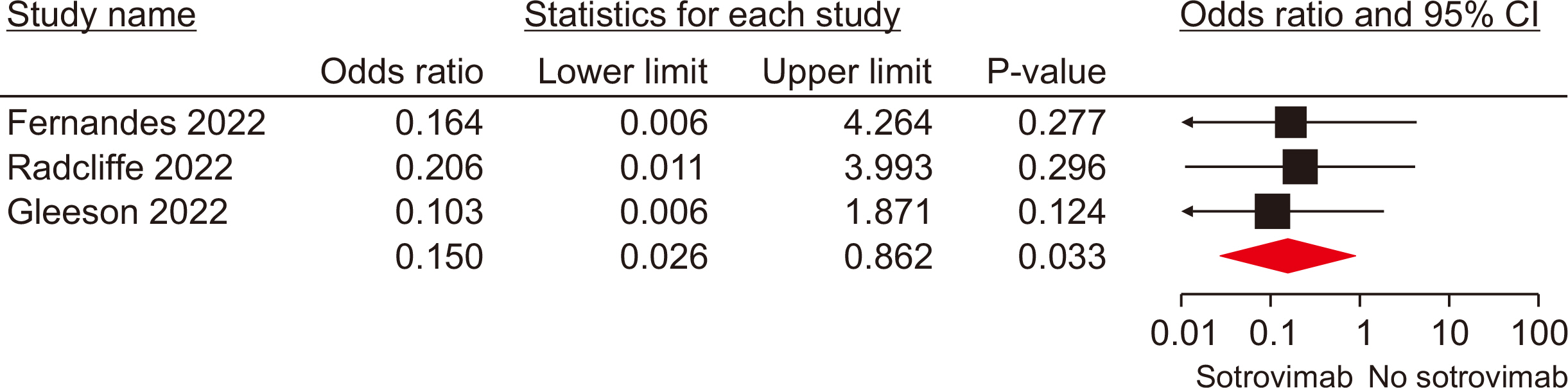
Ten studies, involving a total of 1,569 patients, were included. The meta-analysis revealed significant differences between the patients administered sotrovimab and those treated with the standard of care. These differences were observed in mortality rate (odds ratio [OR], 0.15; 95% confidence interval [CI], 0.03–0.67), hospitalization rate (OR, 0.35; 95% CI, 0.21–0.57), intensive care unit (ICU) admission rate (OR, 0.16; 95% CI, 0.04–0.62), the need for supplemental oxygen therapy (OR, 0.22; 95% CI, 0.09–0.51), and the need for mechanical ventilation (OR, 0.09; 95% CI, 0.01–0.70). However, no signifi-cant difference was observed between sotrovimab and other treatments regarding the rates of hospitalization or ICU admission (P>0.05). Regarding safety, sotrovimab was associated with a lower rate of adverse events compared to the absence of sotrovimab (OR, 0.15; 95% CI, 0.02–0.86).
Conclusions
These results suggest that sotrovimab may improve efficacy outcomes among SOTRs with COVID-19. Nevertheless, additional high-quality trials are necessary to confirm these findings.
Keyword
Figure
Cited by 1 articles
-
Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis
Saeed Khorramnia, Zia Navidi, Amirhossein Orandi, Mojgan Mohajeri Iravani, Amirali Orandi, Ebadallah Shiri Malekabad, Seyed Hamid Pakzad Moghadam
Clin Transplant Res. 2024;38(2):136-144. doi: 10.4285/ctr.24.0015.
Reference
-
1. Jering KS, McGrath MM, Mc Causland FR, Claggett B, Cunningham JW, Solomon SD. 2022; Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant. 36:e14492. DOI: 10.1111/ctr.14492. PMID: 34558116. PMCID: PMC8646895.2. Peghin M, Graziano E, Grossi PA. 2022; SARS-CoV-2 vaccination in solid-organ transplant recipients. Vaccines (Basel). 10:1430. DOI: 10.3390/vaccines10091430. PMID: 36146506. PMCID: PMC9503203.3. Dhand A, Lobo SA, Wolfe K, Feola N, Nabors C. 2021; Bamlanivimab for treatment of COVID-19 in solid organ transplant recipients: early single-center experience. Clin Transplant. 35:e14245. DOI: 10.1111/ctr.14245. PMID: 33595145. PMCID: PMC7995073.4. Hedvat J, Lange NW, Salerno DM, DeFilippis EM, Kovac D, Corbo H, et al. 2022; COVID-19 therapeutics and outcomes among solid organ transplant recipients during the Omicron BA.1 era. Am J Transplant. 22:2682–8. DOI: 10.1111/ajt.17140. PMID: 35801839. PMCID: PMC9349644.5. An EUA for bamlanivimab - a monoclonal antibody for COVID-19. Med Lett Drugs Ther. 2020; 62:185–6.6. An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther. 2020; 62:201–2.7. Gueguen J, Colosio C, Del Bello A, Scemla A, N'Guyen Y, Rouzaud C, et al. 2022; Early administration of anti-SARS-CoV-2 monoclonal antibodies prevents severe COVID-19 in kidney transplant patients. Kidney Int Rep. 7:1241–7. DOI: 10.1016/j.ekir.2022.03.020. PMID: 35372734. PMCID: PMC8957354.8. Chavarot N, Melenotte C, Amrouche L, Rouzaud C, Sberro-Soussan R, Pavie J, et al. 2022; Early treatment with sotrovimab monoclonal antibody in kidney transplant recipients with Omicron infection. Kidney Int. 101:1290–3. DOI: 10.1016/j.kint.2022.04.003. PMID: 35421508. PMCID: PMC9001009.9. Yetmar ZA, Beam E, O'Horo JC, Seville MT, Brumble L, Ganesh R, et al. 2022; Outcomes of bebtelovimab and sotrovimab treatment of solid organ transplant recipients with mild-to-moderate coronavirus disease 2019 during the Omicron epoch. Transpl Infect Dis. 24:e13901. DOI: 10.1111/tid.13901. PMID: 35848574. PMCID: PMC9349935.10. Radcliffe C, Palacios CF, Azar MM, Cohen E, Malinis M. 2022; Real-world experience with available, outpatient COVID-19 therapies in solid organ transplant recipients during the omicron surge. Am J Transplant. 22:2458–63. DOI: 10.1111/ajt.17098. PMID: 35583664. PMCID: PMC9348251.11. Solera JT, Arbol BG, Alshahrani A, Bahinskaya I, Marks N, Humar A, et al. 2022; Impact of vaccination and early monoclonal antibody therapy on coronavirus disease 2019 outcomes in organ transplant recipients during the Omicron wave. Clin Infect Dis. 75:2193–200. DOI: 10.1093/cid/ciac324. PMID: 35445690. PMCID: PMC9278130.12. Birnie E, Biemond JJ, Appelman B, de Bree GJ, Jonges M, Welkers MR, et al. 2022; Development of resistance-associated mutations after sotrovimab administration in high-risk individuals infected with the SARS-CoV-2 Omicron variant. JAMA. 328:1104–7. DOI: 10.1001/jama.2022.13854. PMID: 35913747. PMCID: PMC9344387.13. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. 2015; Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 4:1. DOI: 10.1186/2046-4053-4-1. PMID: 25554246. PMCID: PMC4320440.14. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. 2016; ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. DOI: 10.1136/bmj.i4919. PMID: 27733354. PMCID: PMC5062054.15. Casutt A, Papadimitriou-Olivgeris M, Ioakeim F, Aubert JD, Manuel O, Koutsokera A. 2023; Outcomes of SARS-CoV-2 infection among lung transplant recipients: a single center retrospective study. Transpl Infect Dis. 25:e14007. DOI: 10.1111/tid.14007. PMID: 36602439.16. Fernandes G, Devresse A, Scohy A, De Greef J, Yombi JC, Belkhir L, et al. 2022; Monoclonal antibody therapy in kidney transplant recipients with Delta and Omicron variants of SARS-CoV-2: a single-center case series. Kidney Med. 4:100470. DOI: 10.1016/j.xkme.2022.100470. PMID: 35493029. PMCID: PMC9042411.17. Gleeson S, Martin P, Thomson T, Thind A, Prendecki M, Spensley KJ, et al. Kidney transplant recipients and Omicron: outcomes, effect of vaccines and the efficacy and safety of novel treatments. medRxiv [Preprint]. 2022. Available from: https://doi.org/10.1101/2022.05.03.22274524. cited 2023 Sep 16. DOI: 10.1101/2022.05.03.22274524.18. Papadimitriou-Olivgeris M, Cipriano A, Guggisberg N, Kroemer M, Tschopp J, Manuel O, et al. 2022; Outcome of COVID-19 in kidney transplant recipients through the SARS-CoV-2 variants eras: role of anti-SARS-CoV-2 monoclonal antibodies. Transpl Int. 35:10721. DOI: 10.3389/ti.2022.10721. PMID: 36267693. PMCID: PMC9576844.19. Wong G, Rowlandson M, Sabanayagam D, Ginn AN, Kable K, Sciberras F, et al. 2022; COVID-19 infection with the omicron SARS-CoV-2 variant in a cohort of kidney and kidney pancreas transplant recipients: clinical features, risk factors, and outcomes. Transplantation. 106:1860–6. DOI: 10.1097/TP.0000000000004203. PMID: 35675438.20. Vellas C, Trémeaux P, Del Bello A, Latour J, Jeanne N, Ranger N, et al. 2022; Resistance mutations in SARS-CoV-2 omicron variant in patients treated with sotrovimab. Clin Microbiol Infect. 28:1297–9. DOI: 10.1016/j.cmi.2022.05.002. PMID: 35595125. PMCID: PMC9112603.21. US Food and Drug Administration (FDA). 2022. FDA updates Sotrovimab emergency use authorization [Internet]. FDA;Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-sotrovimab-emergency-use-authorization. cited 2023 Sep 16.22. Farhadian N, Farhadian M, Zamanian MH, Taghadosi M, Vaziri S. 2023; Sotrovimab therapy in solid organ transplant recipients with mild to moderate COVID-19: a systematic review and meta-analysis. Immunopharmacol Immunotoxicol. 45:402–8. DOI: 10.1080/08923973.2022.2160733. PMID: 36537311.23. Amani B, Amani B. 2022; Efficacy and safety of sotrovimab in patients with COVID-19: a rapid review and meta-analysis. Rev Med Virol. 32:e2402. DOI: 10.1002/rmv.2402. PMID: 36226323. PMCID: PMC9874927.24. Yang M, Li T, Wang Y, Tran C, Zhao S, Ao G. 2022; Monoclonal antibody therapy improves severity and mortality of COVID-19 in organ transplant recipients: a meta-analysis. J Infect. 85:436–80. DOI: 10.1016/j.jinf.2022.06.027. PMCID: PMC9247112.25. Wu WL, Chiang CY, Lai SC, Yu CY, Huang YL, Liao HC, et al. 2022; Monoclonal antibody targeting the conserved region of the SARS-CoV-2 spike protein to overcome viral variants. JCI Insight. 7:e157597. DOI: 10.1172/jci.insight.157597. PMID: 35290246. PMCID: PMC9089791.26. Al-Obaidi MM, Gungor AB, Nematollahi S, Zangeneh TT, Bedrick EJ, Johnson KM, et al. 2022; Effectiveness of casirivimab-imdevimab monoclonal antibody treatment among high-risk patients with severe acute respiratory syndrome coronavirus 2 B.1.617.2 (Delta variant) infection. Open Forum Infect Dis. 9:ofac186. DOI: 10.1093/ofid/ofac186. PMID: 35791354. PMCID: PMC9047202.27. Ganesh R, Pawlowski CF, O'Horo JC, Arndt LL, Arndt RF, Bell SJ, et al. 2021; Intravenous bamlanivimab use associates with reduced hospitalization in high-risk patients with mild to moderate COVID-19. J Clin Invest. 131:e151697. DOI: 10.1172/JCI151697. PMID: 34411003. PMCID: PMC8483756.28. Kim T, Joo DH, Lee SW, Lee J, Lee SJ, Kang J. 2022; Real-world efficacy of regdanvimab on clinical outcomes in patients with mild to moderate COVID-19. J Clin Med. 11:1412. DOI: 10.3390/jcm11051412. PMID: 35268503. PMCID: PMC8911404.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The first case of brain death organ donation in a positive COVID-19 donors in Korea
- Remdesivir in solid organ transplant recipients with COVID-19: a systematic review and meta-analysis
- Tixagevimab/cilgavimab prophylaxis against COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis
- SARS-CoV-2 vaccine-elicited immune responses in solid organ transplant recipients
- Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumonia