Korean J Pain.
2023 Jul;36(3):335-346. 10.3344/kjp.23039.
Perampanel ameliorates nitroglycerin-induced migraine through inhibition of the cAMP/ PKA/CREB signaling pathway in the trigeminal ganglion in rats
- Affiliations
-
- 1Department of Neurology, First Affiliated Hospital of Harbin Medical University, Harbin, China
- 2Department of Neurology, The Third Hospital of Jinan Shandong, Jinan, China
- 3Department of Neurology, Shengli Oilfield Central Hospital, Shandong, China
- 4Department of Neurology, Binzhou Medical University Hospital, Shandong, China
- KMID: 2544245
- DOI: http://doi.org/10.3344/kjp.23039
Abstract
- Background
Perampanel, a highly selective glutamate AMPA receptor antagonist, is widely used to treat epilepsy. Since the existence of common pathophysiological features between epilepsy and migraine, the aim of this study was to investigate whether perampanel could exert an antimigraine effect.
Methods
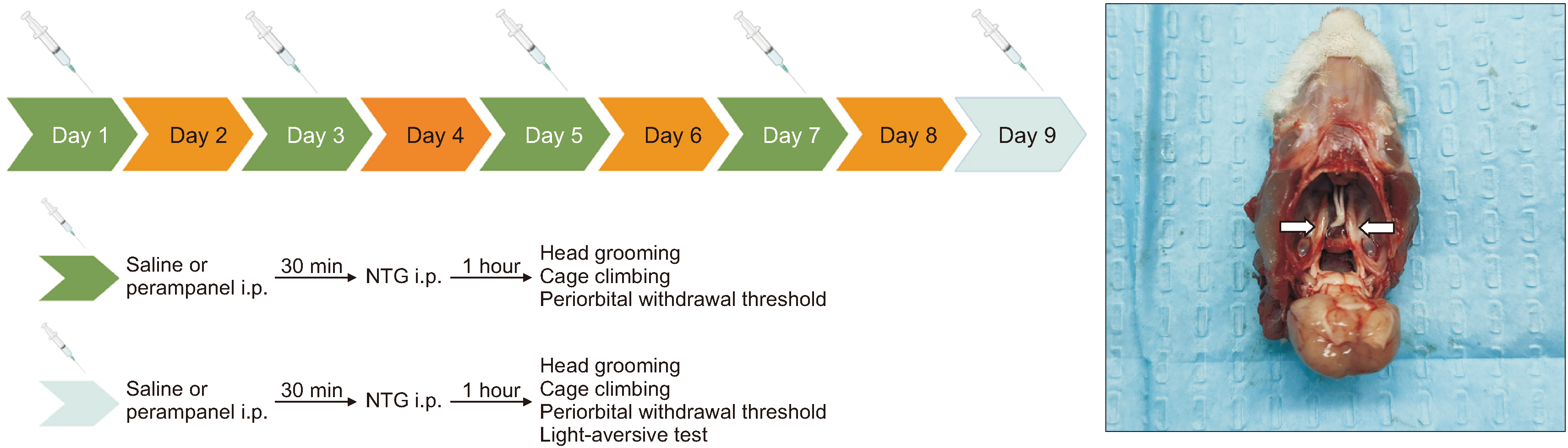
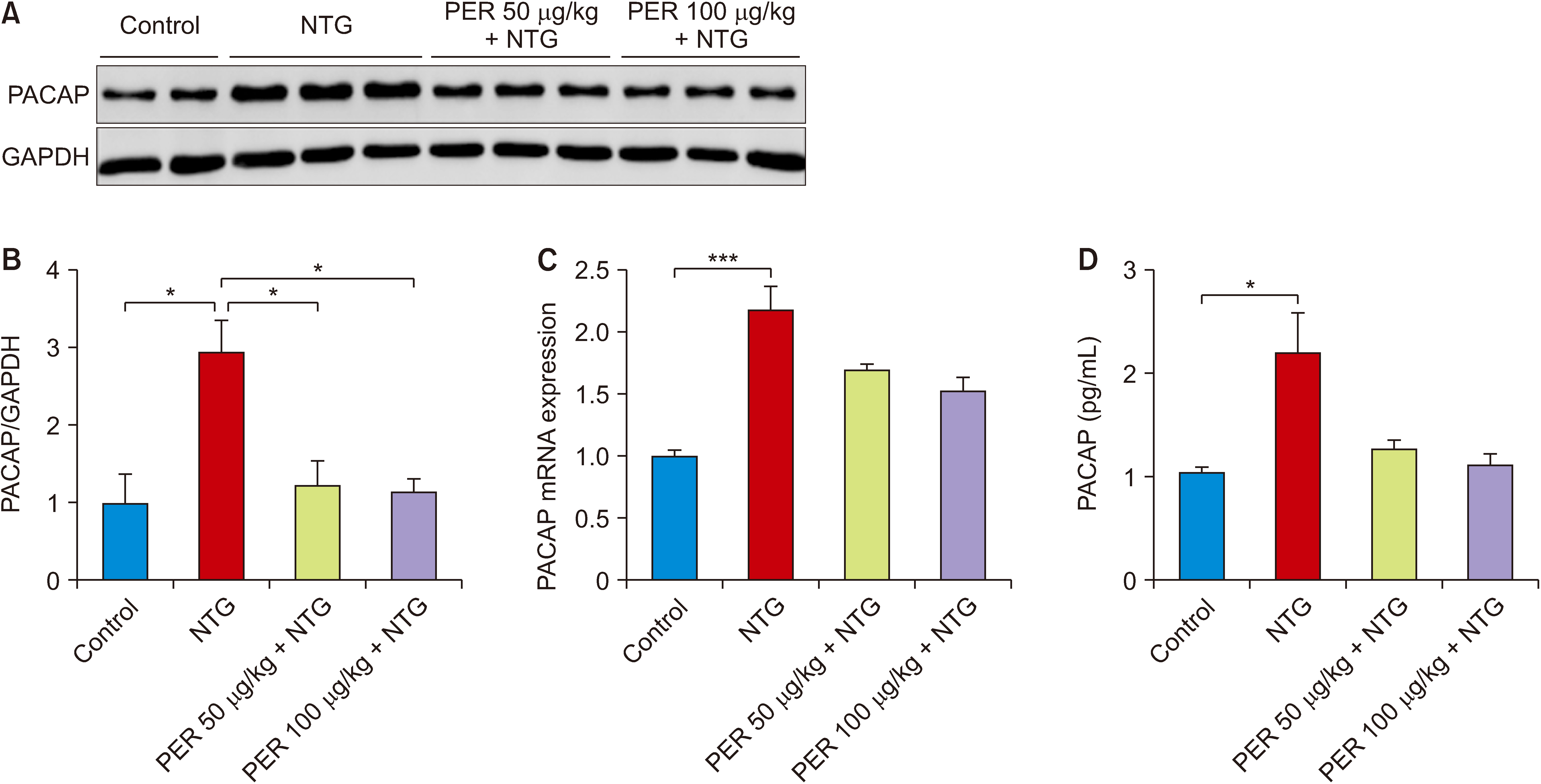
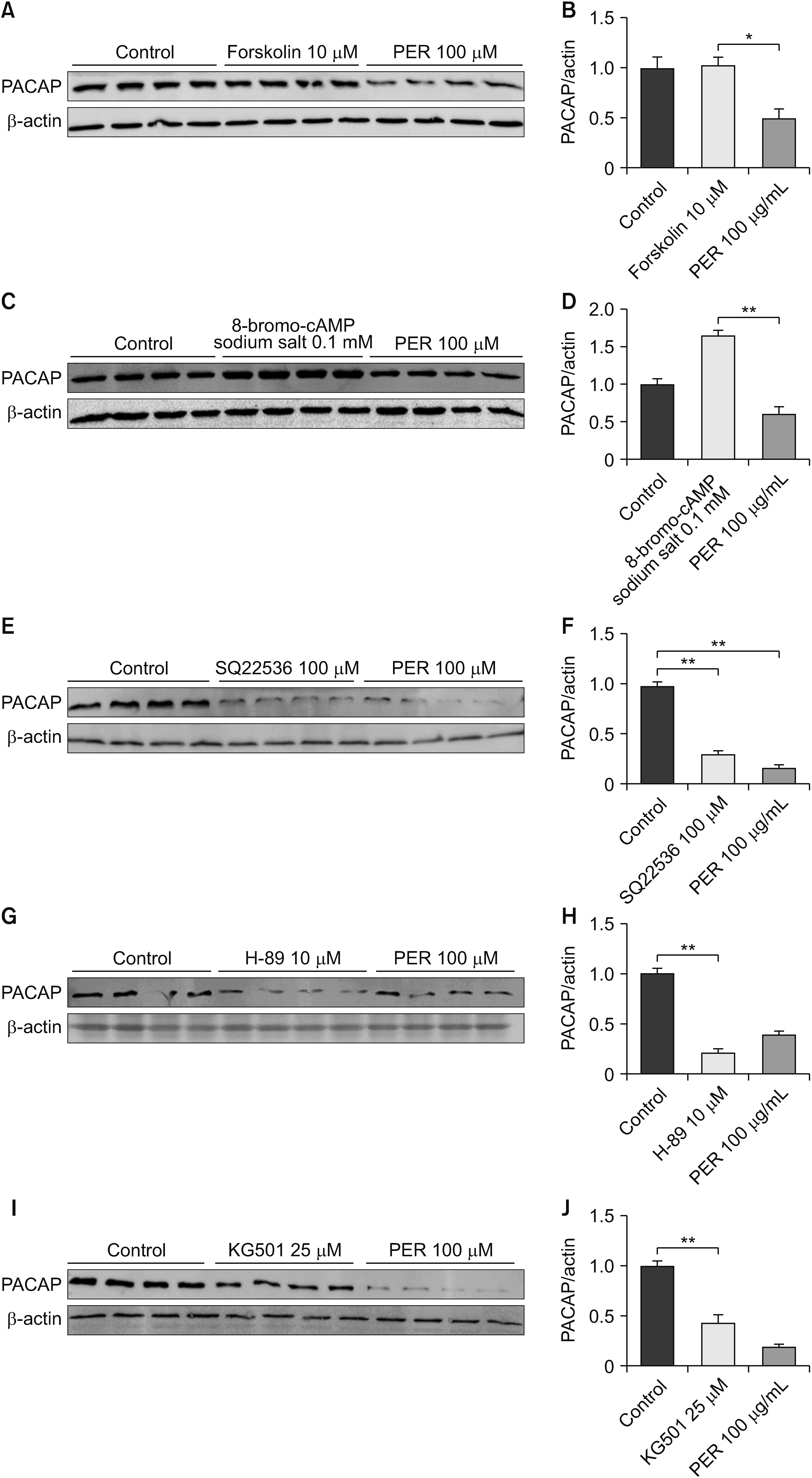
Nitroglycerin (NTG) was used to induce a migraine model in rats, and the model animals were pretreatment with 50 μg/kg and 100 μg/kg perampanel. The expression of pituitary adenylate-cyclase-activating polypeptide (PACAP) was quantified by western blot and quantitative real-time PCR in the trigeminal ganglion, and rat-specific enzyme-linked immunosorbent assay in serum. Western blot was also conducted to explore the effects of perampanel treatment on the phospholipase C (PLC)/protein kinase C (PKC) and protein kinase A (PKA)/ cAMP-responsive-element-binding protein (CREB) signaling pathways. Moreover, the cAMP/PKA/CREB-dependent mechanism was evaluated via in vitro stimulation of hippocampal neurons. The cells were treated with perampanel, antagonists and agonists for 24 hours and cell lysates were prepared for western blot analysis.
Results
Perampanel treatment notably increased the mechanical withdrawal threshold and decreased head grooming and light-aversive behaviors in NTG-treated rats. It also decreased PACAP expression and affected cAMP/ PKA/CREB signaling pathway. However, PLC/PKC signaling pathway may not be involved in this treatment. In in vitro studies, perampanel notably decreased PACAP expression by inhibiting cAMP/PKA/CREB signaling pathway.
Conclusions
This study shows that perampanel inhibits the migraine-like pain response and that this beneficial effect might be attributable to regulation of the cAMP/PKA/CREB signaling pathway.
Keyword
Figure
Cited by 1 articles
-
Data sharing and research integrity: lessons from a recent retraction
Francis Sahngun Nahm
Korean J Pain. 2025;38(1):1-3. doi: 10.3344/kjp.23220r.
Reference
-
1. Global Burden of Disease Study 2013 Collaborators. 2015; Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 386:743–800. DOI: 10.1016/S0140-6736(15)60692-4. PMID: 26063472. PMCID: PMC4561509.2. GBD 2015 Neurological Disorders Collaborator Group. 2017; Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 16:877–97. DOI: 10.1016/S1474-4422(17)30299-5. PMID: 28931491. PMCID: PMC5641502.3. 2018; Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38:1–211. DOI: 10.1177/0333102417738202. PMID: 29368949.4. Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. 2014; Characterization of a novel model of chronic migraine. Pain. 155:269–74. DOI: 10.1016/j.pain.2013.10.004. PMID: 24121068. PMCID: PMC3920577.
Article5. Tuka B, Szabó N, Tóth E, Kincses ZT, Párdutz Á, Szok D, et al. 2016; Release of PACAP-38 in episodic cluster headache patients - an exploratory study. J Headache Pain. 17:69. DOI: 10.1186/s10194-016-0660-7. PMID: 27475101. PMCID: PMC4967416.
Article6. Amin FM, Hougaard A, Schytz HW, Asghar MS, Lundholm E, Parvaiz AI, et al. 2014; Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain. 137:779–94. DOI: 10.1093/brain/awt369. PMID: 24501094.
Article7. Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, et al. 2012; Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 45:633–44. DOI: 10.1016/j.nbd.2011.10.010. PMID: 22033344.
Article8. Hanada T, Hashizume Y, Tokuhara N, Takenaka O, Kohmura N, Ogasawara A, et al. 2011; Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 52:1331–40. DOI: 10.1111/j.1528-1167.2011.03109.x. PMID: 21635236.
Article9. Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. 1996; Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 16:1179–88. DOI: 10.1016/S0896-6273(00)80144-0. PMID: 8663994.
Article10. Nye BL, Thadani VM. 2015; Migraine and epilepsy: review of the literature. Headache. 55:359–80. DOI: 10.1111/head.12536. PMID: 25754865.
Article11. Chan K, MaassenVanDenBrink A. 2014; Glutamate receptor antagonists in the management of migraine. Drugs. 74:1165–76. DOI: 10.1007/s40265-014-0262-0. PMID: 25030431.
Article12. Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, et al. 2008; Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol Pain. 4:67. DOI: 10.1186/1744-8069-4-67. PMID: 19116032. PMCID: PMC2628655. PMID: 549e0037c15b4a679e8f4cc0f1081361.
Article13. Tringali G, Currò D, Navarra P. 2018; Perampanel inhibits calcitonin gene-related peptide release from rat brainstem in vitro. J Headache Pain. 19:107. DOI: 10.1186/s10194-018-0940-5. PMID: 30419806. PMCID: PMC6755590. PMID: f5224fb2b3904254b4393801e9c75cc7.
Article14. Villalón CM, Olesen J. 2009; The role of CGRP in the pathophysiology of migraine and efficacy of CGRP receptor antagonists as acute antimigraine drugs. Pharmacol Ther. 124:309–23. DOI: 10.1016/j.pharmthera.2009.09.003. PMID: 19796656.
Article15. Hara K, Haranishi Y, Terada T. 2020; Intrathecally administered perampanel alleviates neuropathic and inflammatory pain in rats. Eur J Pharmacol. 872:172949. DOI: 10.1016/j.ejphar.2020.172949. PMID: 31991141.
Article16. Askari-Zahabi K, Abbasnejad M, Kooshki R, Esmaeili-Mahani S. 2021; Orexin one receptors within the basolateral amygdala are involved in the modulation of cognitive deficits associated with a migraine-like state in rats. Neurol Res. 43:1087–97. DOI: 10.1080/01616412.2021.1949687. PMID: 34233602.
Article17. Mahmoudi J, Mohaddes G, Erfani M, Sadigh-Eteghad S, Karimi P, Rajabi M, et al. 2018; Cerebrolysin attenuates hyperalgesia, photophobia, and neuroinflammation in a nitroglycerin-induced migraine model in rats. Brain Res Bull. 140:197–204. DOI: 10.1016/j.brainresbull.2018.05.008. PMID: 29752991.
Article18. Tang Y, Liu S, Shu H, Xing Y, Tao F. 2018; AMPA receptor GluA1 Ser831 phosphorylation is critical for nitroglycerin-induced migraine-like pain. Neuropharmacology. 133:462–9. DOI: 10.1016/j.neuropharm.2018.02.026. PMID: 29486167. PMCID: PMC5858972.
Article19. Mustelin L, Raevuori A, Kaprio J, Keski-Rahkonen A. 2014; Association between eating disorders and migraine may be explained by major depression. Int J Eat Disord. 47:884–7. DOI: 10.1002/eat.22311. PMID: 24888633.
Article20. Wang K, Zhai Q, Wang S, Li Q, Liu J, Meng F, et al. 2021; Cryptotanshinone ameliorates CUS-induced depressive-like behaviors in mice. Transl Neurosci. 12:469–81. DOI: 10.1515/tnsci-2020-0198. PMID: 34900345. PMCID: PMC8633587. PMID: c89ef6bd6b7d4da991f44e4049713ab2.
Article21. Liu L, Zheng J, Huang XF, Zhu X, Ding SM, Ke HM, et al. 2018; The neuroprotective and antidepressant-like effects of Hcyb1, a novel selective PDE2 inhibitor. CNS Neurosci Ther. 24:652–60. DOI: 10.1111/cns.12863. PMID: 29704309. PMCID: PMC6489804.
Article22. Carruthers AM, Sellers LA, Jenkins DW, Jarvie EM, Feniuk W, Humphrey PP. 2001; Adenosine A(1) receptor-mediated inhibition of protein kinase A-induced calcitonin gene-related peptide release from rat trigeminal neurons. Mol Pharmacol. 59:1533–41. DOI: 10.1124/mol.59.6.1533. PMID: 11353815.
Article23. Baratloo A, Mirbaha S, Delavar Kasmaei H, Payandemehr P, Elmaraezy A, Negida A. 2017; Intravenous caffeine citrate vs. magnesium sulfate for reducing pain in patients with acute migraine headache; a prospective quasi-experimental study. Korean J Pain. 30:176–82. DOI: 10.3344/kjp.2017.30.3.176. PMID: 28757917. PMCID: PMC5532524.
Article24. Casili G, Lanza M, Filippone A, Campolo M, Paterniti I, Cuzzocrea S, et al. 2020; Dimethyl fumarate alleviates the nitroglycerin (NTG)-induced migraine in mice. J Neuroinflammation. 17:59. DOI: 10.1186/s12974-020-01736-1. PMID: 32066464. PMCID: PMC7469611. PMID: 489394dbd10c4f849f6c6eaa63a1fa31.
Article25. Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltrán E, et al. European Headache Federation School of Advanced Studies (EHF-SAS). 2017; Blocking CGRP in migraine patients - a review of pros and cons. J Headache Pain. 18:96. DOI: 10.1186/s10194-017-0807-1. PMID: 28948500. PMCID: PMC5612904. PMID: d2ba45670bee4a83b7dd02bf83f5592e.
Article26. Goadsby PJ, Lipton RB, Ferrari MD. 2002; Migraine--current understanding and treatment. N Engl J Med. 346:257–70. DOI: 10.1056/NEJMra010917. PMID: 11807151.27. Han X, Ran Y, Su M, Liu Y, Tang W, Dong Z, et al. 2017; Chronic changes in pituitary adenylate cyclase-activating polypeptide and related receptors in response to repeated chemical dural stimulation in rats. Mol Pain. 13:1744806917720361. DOI: 10.1177/1744806917720361. PMID: 28776455. PMCID: PMC5546650.
Article28. Brewerton TD, George MS. 1993; Is migraine related to the eating disorders? Int J Eat Disord. 14:75–9. DOI: 10.1002/1098-108X(199307)14:1<75::AID-EAT2260140110>3.0.CO;2-D. PMID: 8339102.
Article29. D'Andrea G, Ostuzzi R, Bolner A, Colavito D, Leon A. 2012; Is migraine a risk factor for the occurrence of eating disorders? Prevalence and biochemical evidences. Neurol Sci. 33 Suppl 1:S71–6. DOI: 10.1007/s10072-012-1045-6. PMID: 22644175.30. Catterall WA. 2015; Regulation of cardiac calcium channels in the fight-or-flight response. Curr Mol Pharmacol. 8:12–21. DOI: 10.2174/1874467208666150507103417. PMID: 25966697. PMCID: PMC4664455.
Article31. Ahuja M, Jha A, Maléth J, Park S, Muallem S. 2014; cAMP and Ca²+ signaling in secretory epithelia: crosstalk and synergism. Cell Calcium. 55:385–93. DOI: 10.1016/j.ceca.2014.01.006. PMID: 24613710. PMCID: PMC4058382.
Article32. Chen T, Koga K, Descalzi G, Qiu S, Wang J, Zhang LS, et al. 2014; Postsynaptic potentiation of corticospinal projecting neurons in the anterior cingulate cortex after nerve injury. Mol Pain. 10:33. DOI: 10.1186/1744-8069-10-33. PMID: 24890933. PMCID: PMC4060852.
Article33. Yue X, Tumati S, Navratilova E, Strop D, St John PA, Vanderah TW, et al. 2008; Sustained morphine treatment augments basal CGRP release from cultured primary sensory neurons in a Raf-1 dependent manner. Eur J Pharmacol. 584:272–7. DOI: 10.1016/j.ejphar.2008.02.013. PMID: 18328477. PMCID: PMC2375088.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diarylpropionitrile inhibits melanogenesis via protein kinase A/cAMP-response element-binding protein/microphthalmiaassociated transcription factor signaling pathway in α-MSHstimulated B16F10 melanoma cells
- cAMP signaling increases histone deacetylase 8 expression via the Epac2–Rap1A–Akt pathway in H1299 lung cancer cells
- Polydeoxyribonucleotide Ameliorates Inflammation and Apoptosis in Achilles Tendon-Injury Rats
- MSK1 regulates RANKL-induced NFATc1 expression through CREB and c-Fos
- Parathyroid Hormone-Related Protein Promotes the Proliferation of Patient-Derived Glioblastoma Stem Cells via Activating cAMP/PKA Signaling Pathway