Korean J Physiol Pharmacol.
2022 Mar;26(2):113-123. 10.4196/kjpp.2022.26.2.113.
Diarylpropionitrile inhibits melanogenesis via protein kinase A/cAMP-response element-binding protein/microphthalmiaassociated transcription factor signaling pathway in α-MSHstimulated B16F10 melanoma cells
- Affiliations
-
- 1Department of Cosmetics Engineering, Konkuk University, Seoul 05029, Korea
- KMID: 2526730
- DOI: http://doi.org/10.4196/kjpp.2022.26.2.113
Abstract
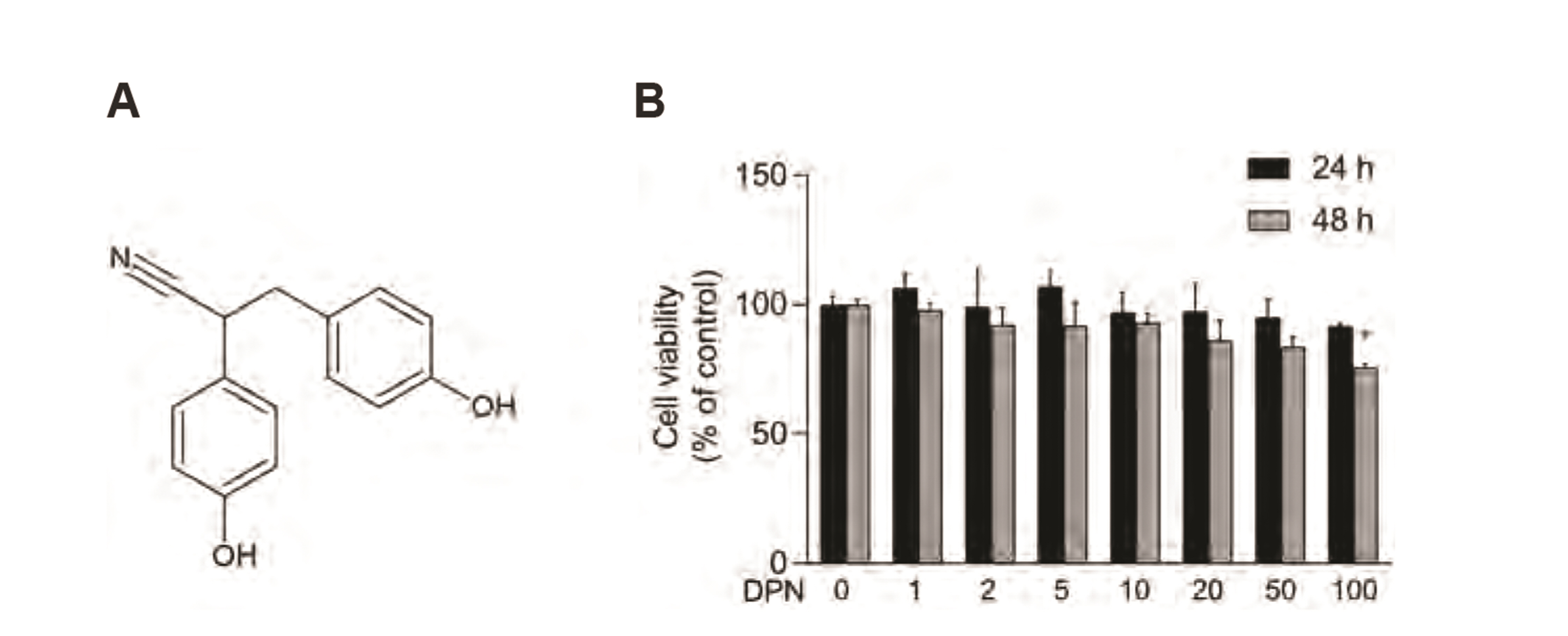
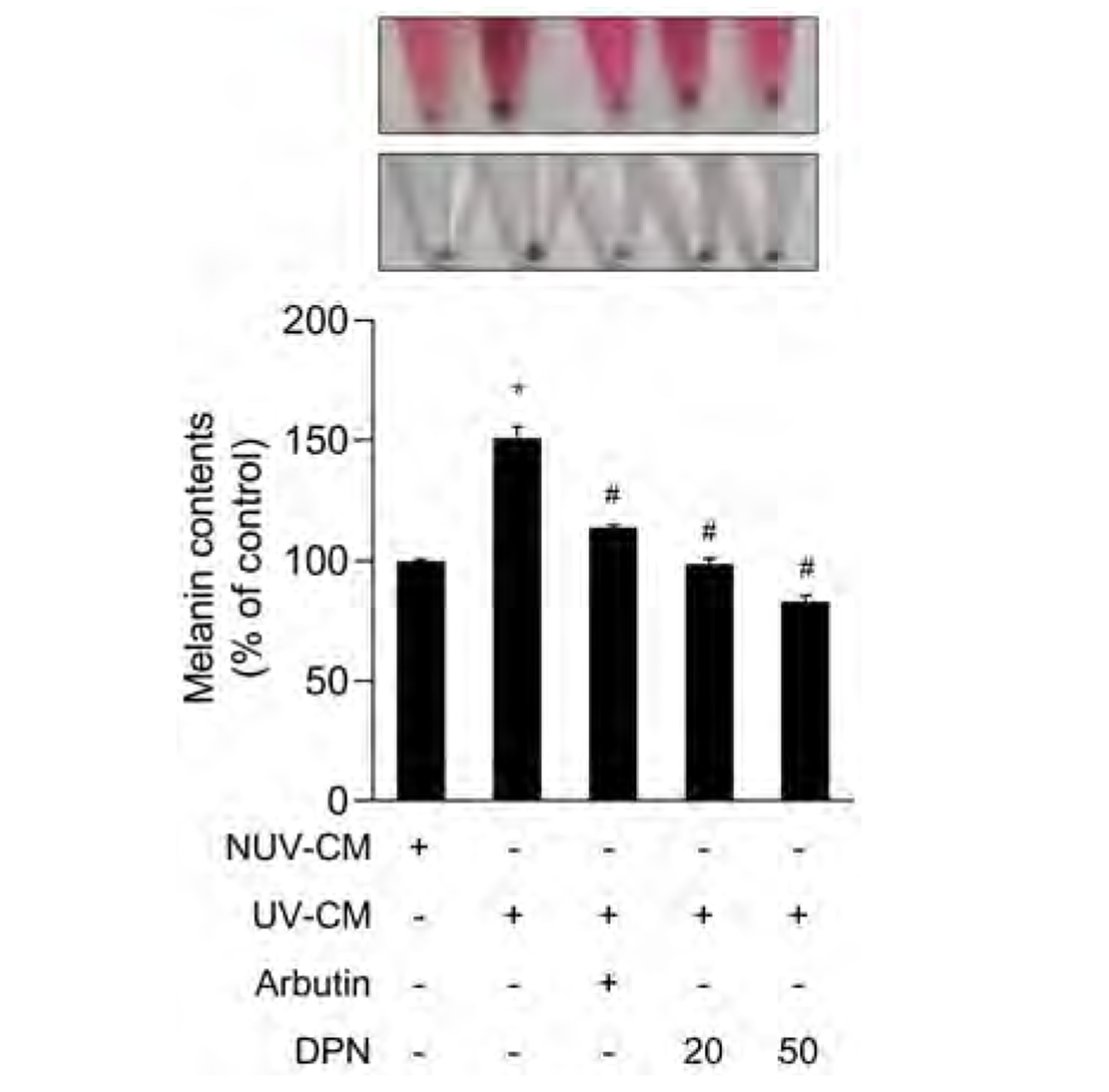
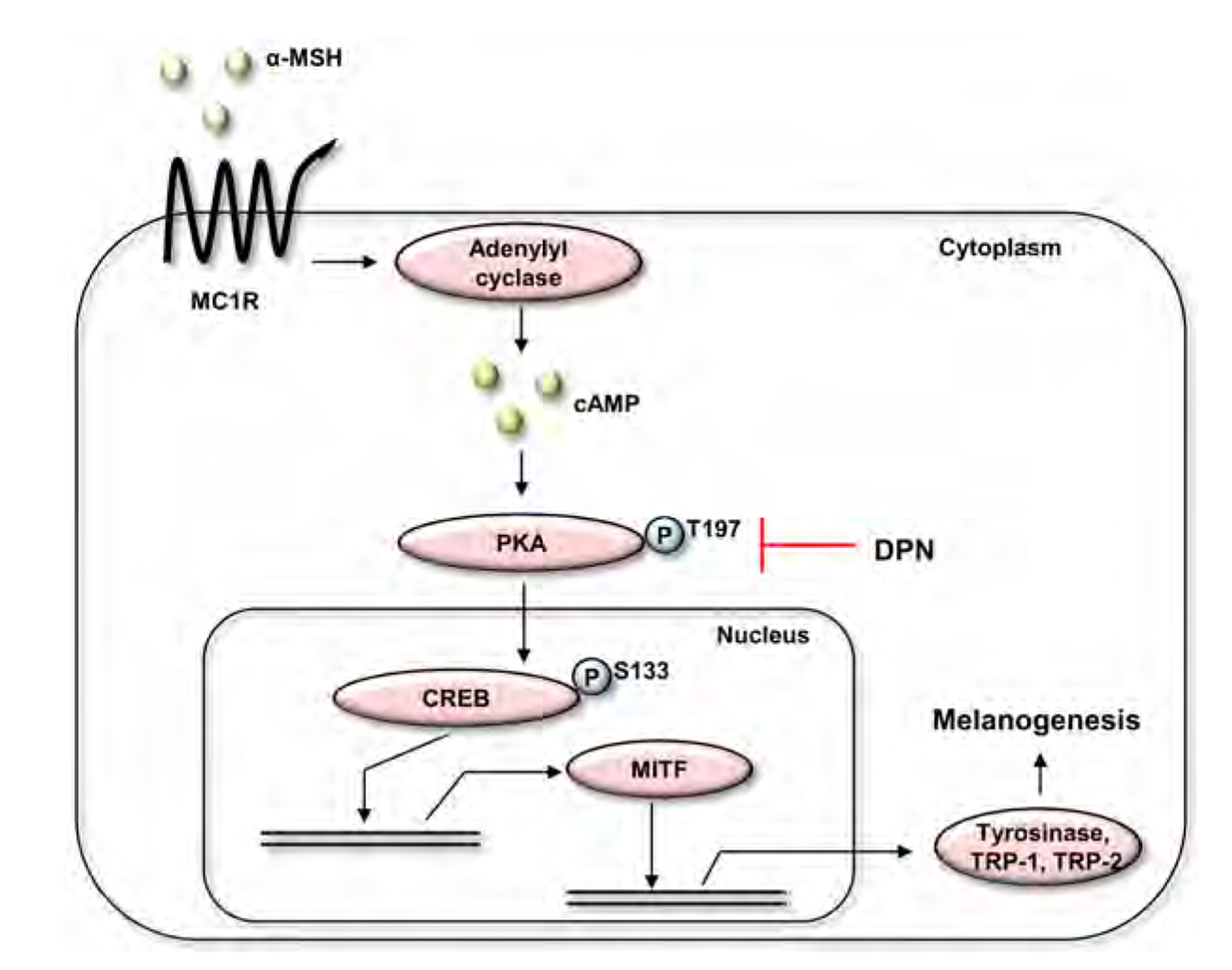
- Diarylpropionitrile (DPN), a selective agonist for estrogen receptor β(ERβ), has been reported to regulate various hormonal responses through activation of ERβ in tissues including the mammary gland and brain. However, the effect of DPN on melanogenesis independent of ERβ has not been studied. The aim of this study is to examine the possibility of anti-melanogenic effect of DPN and its underlying mechanism. Melanin contents and cellular tyrosinase activity assay indicated that DPN inhibited melanin biosynthesis in alpha-melanocyte stimulating hormonestimulated B16F10 melanoma cell line. However, DPN had no direct influence on in vitro tyrosinase catalytic activity. On the other hand, 17β-estradiol had no effect on inhibition of melanogenesis, suggesting that the DPN-mediated suppression of melanin production was not related with estrogen signaling pathway. Immunoblotting analysis showed that DPN down-regulated the expression of microphthalmiaassociated transcription factor (MITF), a central transcription factor of melanogenesis and its down-stream genes including tyrosinase, tyrosinase-related protein (TRP)-1, and TRP-2. Also, DPN attenuated the phosphorylation of protein kinase A (PKA) and cAMP-response element-binding protein (CREB). Additionally, DPN suppressed the melanin synthesis in UVB-irradiated HaCaT conditioned media culture system suggesting that DPN has potential as an anti-melanogenic activity in physiological conditions. Collectively, our data show that DPN inhibits melanogenesis via downregulation of PKA/CREB/MITF signaling pathway.
Keyword
Figure
Reference
-
1. Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. 2001; Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 44:4230–4251. DOI: 10.1021/jm010254a. PMID: 11708925.
Article2. Walf AA, Koonce CJ, Frye CA. 2008; Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol Learn Mem. 89:513–521. DOI: 10.1016/j.nlm.2008.01.008. PMID: 18313947. PMCID: PMC2424257.
Article3. Motylewska E, Stasikowska O, Mełeń-Mucha G. 2009; The inhibitory effect of diarylpropionitrile, a selective agonist of estrogen receptor beta, on the growth of MC38 colon cancer line. Cancer Lett. 276:68–73. DOI: 10.1016/j.canlet.2008.10.050. PMID: 19101081.
Article4. Huang SY, Xin H, Sun J, Li R, Zhang XM, Zhao D. 2013; Estrogen receptor β agonist diarylpropionitrile inhibits lipopolysaccharide-induced regulated on activation normal T cell expressed and secreted (RANTES) production in macrophages by repressing nuclear factor κB activation. Fertil Steril. 100:234–240. DOI: 10.1016/j.fertnstert.2013.02.052. PMID: 23557759.
Article5. Weiser MJ, Wu TJ, Handa RJ. 2009; Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 150:1817–1825. DOI: 10.1210/en.2008-1355. PMID: 19074580. PMCID: PMC2659273.
Article6. Mancuso M, Leonardi S, Giardullo P, Pasquali E, Borra F, Stefano ID, Prisco MG, Tanori M, Scambia G, Majo VD, Pazzaglia S, Saran A, Gallo D. 2011; The estrogen receptor beta agonist diarylpropionitrile (DPN) inhibits medulloblastoma development via anti-proliferative and pro-apototic pathways. Cancer Lett. 308:197–202. DOI: 10.1016/j.canlet.2011.05.004. PMID: 21636213.
Article7. Michel T, Halabalaki M, Skaltsounis AL. 2013; New concepts, experimental approaches, and dereplication strategies for the discovery of novel phytoestrogens from natural sources. Planta Med. 79:514–532. DOI: 10.1055/s-0032-1328300. PMID: 23479392.
Article8. Yang J, Wen L, Jiang Y, Yang B. 2019; Natural estrogen receptor modulators and their heterologous biosynthesis. Trends Endocrinol Metab. 30:66–76. DOI: 10.1016/j.tem.2018.11.002. PMID: 30527917.
Article9. Pillaiyar T, Namasivayam V, Manickam M, Jung SH. 2018; Inhibitors of melanogenesis: an updated review. J Med Chem. 61:7395–7418. DOI: 10.1021/acs.jmedchem.7b00967. PMID: 29763564.
Article10. D'Mello SA, Finlay GJ, Baguley BC, Askarian-Amiri ME. 2016; Signaling pathways in melanogenesis. Int J Mol Sci. 17:1144. DOI: 10.3390/ijms17071144. PMID: 27428965. PMCID: PMC4964517.11. Vachtenheim J, Borovanský J. 2010; "Transcription physiology" of pigment formation in melanocytes: central role of MITF. Exp Dermatol. 19:617–627. DOI: 10.1111/j.1600-0625.2009.01053.x. PMID: 20201954.
Article12. Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ. 1992; A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 11:519–526. DOI: 10.1002/j.1460-2075.1992.tb05082.x. PMID: 1537333. PMCID: PMC556482.
Article13. Kobayashi T, Urabe K, Winder A, Jiménez-Cervantes C, Imokawa G, Brewington T, Solano F, García-Borrón JC, Hearing VJ. 1994; Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 13:5818–5825. DOI: 10.1002/j.1460-2075.1994.tb06925.x. PMID: 7813420. PMCID: PMC395555.
Article14. Chen T, Zhao B, Liu Y, Wang R, Yang Y, Yang L, Dong C. 2018; MITF-M regulates melanogenesis in mouse melanocytes. J Dermatol Sci. 90:253–262. DOI: 10.1016/j.jdermsci.2018.02.008. PMID: 29496358.
Article15. Du J, Miller AJ, Widlund HR, Horstmann MA, Ramaswamy S, Fisher DE. 2003; MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 163:333–343. DOI: 10.1016/S0002-9440(10)63657-7. PMID: 12819038. PMCID: PMC1868174.
Article16. Aoki H, Moro O. 2002; Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R). Life Sci. 71:2171–2179. DOI: 10.1016/S0024-3205(02)01996-3. PMID: 12204775.
Article17. Tagashira H, Miyamoto A, Kitamura S, Tsubata M, Yamaguchi K, Takagaki K, Imokawa G. 2015; UVB stimulates the expression of endothelin B receptor in human melanocytes via a sequential activation of the p38/MSK1/CREB/MITF pathway which can be interrupted by a French maritime pine bark extract through a direct inactivation of MSK1. PLoS One. 10:e0128678. DOI: 10.1371/journal.pone.0128678. PMID: 26030901. PMCID: PMC4452497.
Article18. Box NF, Terzian T. 2008; The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 21:525–533. DOI: 10.1111/j.1755-148X.2008.00495.x. PMID: 18761658.
Article19. Yamaguchi Y, Coelho SG, Zmudzka BZ, Takahashi K, Beer JZ, Hearing VJ, Miller SA. 2008; Cyclobutane pyrimidine dimer formation and p53 production in human skin after repeated UV irradiation. Exp Dermatol. 17:916–924. DOI: 10.1111/j.1600-0625.2008.00722.x. PMID: 18363705.
Article20. Cui R, Widlund HR, Feige E, Lin JY, Wilensky DL, Igras VE, D'Orazio J, Fung CY, Schanbacher CF, Granter SR, Fisher DE. 2007; Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 128:853–864. DOI: 10.1016/j.cell.2006.12.045. PMID: 17350573.
Article21. Yamamoto H, Yamane T, Iguchi K, Tanaka K, Iddamalgoda A, Unno K, Hoshino M, Takeda A. 2015; Melanin production through novel processing of proopiomelanocortin in the extracellular compartment of the auricular skin of C57BL/6 mice after UV-irradiation. Sci Rep. 5:14579. DOI: 10.1038/srep14579. PMID: 26417724. PMCID: PMC4586518.
Article22. Neary CL, Cho-Chung YS. 2001; Nuclear translocation of the catalytic subunit of protein kinase A induced by an antisense oligonucleotide directed against the RIalpha regulatory subunit. Oncogene. 20:8019–8024. DOI: 10.1038/sj.onc.1204992. PMID: 11753685.
Article23. Yun CY, You ST, Kim JH, Chung JH, Han SB, Shin EY, Kim EG. 2015; p21-activated kinase 4 critically regulates melanogenesis via activation of the CREB/MITF and β-catenin/MITF pathways. J Invest Dermatol. 135:1385–1394. DOI: 10.1038/jid.2014.548. PMID: 25560280.
Article24. Buscà R, Ballotti R. 2000; Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 13:60–69. DOI: 10.1034/j.1600-0749.2000.130203.x. PMID: 10841026.
Article25. Hartman ML, Czyz M. 2015; MITF in melanoma: mechanisms behind its expression and activity. Cell Mol Life Sci. 72:1249–1260. DOI: 10.1007/s00018-014-1791-0. PMID: 25433395. PMCID: PMC4363485.
Article26. Smalley K, Eisen T. 2000; The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and anti-proliferative effects in B16 murine melanoma cells. FEBS Lett. 476:198–202. DOI: 10.1016/S0014-5793(00)01726-9. PMID: 10913613.
Article27. Bae IH, Lee ES, Yoo JW, Lee SH, Ko JY, Kim YJ, Lee TR, Kim DY, Lee CS. 2019; Mannosylerythritol lipids inhibit melanogenesis via suppressing ERK-CREB-MiTF-tyrosinase signalling in normal human melanocytes and a three-dimensional human skin equivalent. Exp Dermatol. 28:738–741. DOI: 10.1111/exd.13836. PMID: 30408247.
Article28. Jung JM, Noh TK, Jo SY, Kim SY, Song Y, Kim YH, Chang SE. 2020; Guanine deaminase in human epidermal keratinocytes contributes to skin pigmentation. Molecules. 25:2637. DOI: 10.3390/molecules25112637. PMID: 32517074. PMCID: PMC7321356. PMID: 9ba4120859fa49b7a048c06e76853036.
Article29. Marzagalli M, Casati L, Moretti RM, Montagnani Marelli M, Limonta P. 2015; Estrogen receptor β agonists differentially affect the growth of human melanoma cell lines. PLoS One. 10:e0134396. DOI: 10.1371/journal.pone.0134396. PMID: 26225426. PMCID: PMC4520550.
Article30. Marzagalli M, Montagnani Marelli M, Casati L, Fontana F, Moretti RM, Limonta P. 2016; Estrogen receptor β in melanoma: from molecular insights to potential clinical utility. Front Endocrinol (Lausanne). 7:140. DOI: 10.3389/fendo.2016.00140. PMID: 27833586. PMCID: PMC5080294.
Article31. Levy C, Khaled M, Fisher DE. 2006; MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 12:406–414. DOI: 10.1016/j.molmed.2006.07.008. PMID: 16899407.
Article32. Niwano T, Terazawa S, Nakajima H, Imokawa G. 2018; The stem cell factor-stimulated melanogenesis in human melanocytes can be abrogated by interrupting the phosphorylation of MSK1: evidence for involvement of the p38/MSK1/CREB/MITF axis. Arch Dermatol Res. 310:187–196. DOI: 10.1007/s00403-018-1816-x. PMID: 29362867.
Article33. Cheng X, Ma Y, Moore M, Hemmings BA, Taylor SS. 1998; Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc Natl Acad Sci U S A. 95:9849–9854. DOI: 10.1073/pnas.95.17.9849. PMID: 9707564. PMCID: PMC21425.
Article34. Saha B, Singh SK, Sarkar C, Bera R, Ratha J, Tobin DJ, Bhadra R. 2006; Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 19:595–605. DOI: 10.1111/j.1600-0749.2006.00348.x. PMID: 17083486.
Article35. Yuan XH, Jin ZH. 2018; Paracrine regulation of melanogenesis. Br J Dermatol. 178:632–639. DOI: 10.1111/bjd.15651. PMID: 28494100.
Article36. Lei MJ, Dong Y, Sun CX, Zhang XH. 2017; Resveratrol inhibits proliferation, promotes differentiation and melanogenesis in HT-144 melanoma cells through inhibition of MEK/ERK kinase pathway. Microb Pathog. 111:410–413. DOI: 10.1016/j.micpath.2017.09.029. PMID: 28919486.
Article37. Park J, Park JH, Suh HJ, Lee IC, Koh J, Boo YC. 2014; Effects of resveratrol, oxyresveratrol, and their acetylated derivatives on cellular melanogenesis. Arch Dermatol Res. 306:475–487. DOI: 10.1007/s00403-014-1440-3. PMID: 24414332.
Article38. Lee YS, Kim HK, Lee KJ, Jeon HW, Cui S, Lee YM, Moon BJ, Kim YH, Lee YS. 2010; Inhibitory effect of glyceollin isolated from soybean against melanogenesis in B16 melanoma cells. BMB Rep. 43:461–467. DOI: 10.5483/BMBRep.2010.43.7.461. PMID: 20663406.
Article39. Shin SH, Lee YM. 2013; Glyceollins, a novel class of soybean phytoalexins, inhibit SCF-induced melanogenesis through attenuation of SCF/c-kit downstream signaling pathways. Exp Mol Med. 45:e17. DOI: 10.1038/emm.2013.20. PMID: 23559126. PMCID: PMC3641398.
Article40. Lee AY, Noh M. 2013; The regulation of epidermal melanogenesis via cAMP and/or PKC signaling pathways: insights for the development of hypopigmenting agents. Arch Pharm Res. 36:792–801. DOI: 10.1007/s12272-013-0130-6. PMID: 23604723.
Article41. Bae-Harboe YS, Park HY. 2012; Tyrosinase: a central regulatory protein for cutaneous pigmentation. J Invest Dermatol. 132:2678–2680. DOI: 10.1038/jid.2012.324. PMID: 23187110.
Article42. Fan M, Zhang G, Hu X, Xu X, Gong D. 2017; Quercetin as a tyrosinase inhibitor: inhibitory activity, conformational change and mechanism. Food Res Int. 100(Pt 1):226–233. DOI: 10.1016/j.foodres.2017.07.010. PMID: 28873682.
Article43. Chen QX, Kubo I. 2002; Kinetics of mushroom tyrosinase inhibition by quercetin. J Agric Food Chem. 50:4108–4112. DOI: 10.1021/jf011378z. PMID: 12083892.
Article44. Ferguson CA, Kidson SH. 1997; The regulation of tyrosinase gene transcription. Pigment Cell Res. 10:127–138. DOI: 10.1111/j.1600-0749.1997.tb00474.x. PMID: 9266599.
Article45. Verastegui C, Bertolotto C, Bille K, Abbe P, Ortonne JP, Ballotti R. 2000; TFE3, a transcription factor homologous to Microphthalmia, is a potential transcriptional activator of tyrosinase and TyrpI genes. Mol Endocrinol. 14:449–456. DOI: 10.1210/mend.14.3.0428. PMID: 10707962.
Article46. Murisier F, Guichard S, Beermann F. 2007; The tyrosinase enhancer is activated by Sox10 and Mitf in mouse melanocytes. Pigment Cell Res. 20:173–184. DOI: 10.1111/j.1600-0749.2007.00368.x. PMID: 17516925.
Article47. Ando H, Ichihashi M, Hearing VJ. 2009; Role of the ubiquitin proteasome system in regulating skin pigmentation. Int J Mol Sci. 10:4428–4434. DOI: 10.3390/ijms10104428. PMID: 20057953. PMCID: PMC2790116.
Article48. Kageyama A, Oka M, Okada T, Nakamura S, Ueyama T, Saito N, Hearing VJ, Ichihashi M, Nishigori C. 2004; Down-regulation of melanogenesis by phospholipase D2 through ubiquitin proteasome-mediated degradation of tyrosinase. J Biol Chem. 279:27774–27780. DOI: 10.1074/jbc.M401786200. PMID: 15067002.
Article49. Widlund HR, Fisher DE. 2003; Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene. 22:3035–3041. DOI: 10.1038/sj.onc.1206443. PMID: 12789278.
Article50. Fang D, Tsuji Y, Setaluri V. 2002; Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 30:3096–3106. DOI: 10.1093/nar/gkf424. PMID: 12136092. PMCID: PMC135745.
Article51. Ludwig A, Rehberg S, Wegner M. 2004; Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors. FEBS Lett. 556:236–244. DOI: 10.1016/S0014-5793(03)01446-7. PMID: 14706856.
Article52. Roh E, Yun CY, Yun JY, Park D, Kim ND, Hwang BY, Jung SH, Park SK, Kim YB, Han SB, Kim Y. 2013; cAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder. J Invest Dermatol. 133:1072–1079. DOI: 10.1038/jid.2012.425. PMID: 23254773.
Article53. Lee HS, Goh MJ, Kim J, Choi TJ, Lee HK, Na YJ, Cho KH. 2015; A systems-biological study on the identification of safe and effective molecular targets for the reduction of ultraviolet B-induced skin pigmentation. Sci Rep. 5:10305. DOI: 10.1038/srep10305. PMID: 25980672. PMCID: PMC4434836.
Article54. Buscà R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychène A, Ortonne JP, Ballotti R. 2000; Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 19:2900–2910. DOI: 10.1093/emboj/19.12.2900. PMID: 10856235. PMCID: PMC203360.
Article55. Ko GA, Cho SK. 2018; Phytol suppresses melanogenesis through proteasomal degradation of MITF via the ROS-ERK signaling pathway. Chem Biol Interact. 286:132–140. DOI: 10.1016/j.cbi.2018.02.033. PMID: 29486182.
Article56. Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, Fisher DZ, Fisher DE. 2000; c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 14:301–312. DOI: 10.1101/gad.14.3.301. PMID: 10673502. PMCID: PMC316361.
Article57. Valero MS, Pereboom D, Barcelo-Batllory S, Brines L, Garay RP, Alda JO. 2011; Protein kinase A signalling is involved in the relaxant responses to the selective β-oestrogen receptor agonist diarylpropionitrile in rat aortic smooth muscle in vitro. J Pharm Pharmacol. 63:222–229. DOI: 10.1111/j.2042-7158.2010.01203.x. PMID: 21235586.
Article58. Dumaz N, Marais R. 2005; Integrating signals between cAMP and the RAS/RAF/MEK/ERK signalling pathways. Based on the anniversary prize of the Gesellschaft für Biochemie und Molekularbiologie Lecture delivered on 5 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 272:3491–3504. DOI: 10.1111/j.1742-4658.2005.04763.x. PMID: 16008550.
Article59. Poletini MO, de Assis LV, Moraes MN, Castrucci AM. 2016; Estradiol differently affects melanin synthesis of malignant and normal melanocytes: a relationship with clock and clock-controlled genes. Mol Cell Biochem. 421:29–39. DOI: 10.1007/s11010-016-2781-3. PMID: 27535239.
Article60. Martin AG, Leal-Khouri S. 1992; Physiologic skin changes associated with pregnancy. Int J Dermatol. 31:375–378. DOI: 10.1111/j.1365-4362.1992.tb02662.x. PMID: 1512085.
Article61. Dika E, Patrizi A, Lambertini M, Manuelpillai N, Fiorentino M, Altimari A, Ferracin M, Lauriola M, Fabbri E, Campione E, Veronesi G, Scarfì F. 2019; Estrogen receptors and melanoma: a review. Cells. 8:1463. DOI: 10.3390/cells8111463. PMID: 31752344. PMCID: PMC6912660. PMID: ccc5d5e6b8ab4e0fa7333651992e1cca.
Article62. Ranson M, Posen S, Mason RS. 1988; Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 91:593–598. DOI: 10.1111/1523-1747.ep12477126. PMID: 2848074.
Article63. Sun M, Xie HF, Tang Y, Lin SQ, Li JM, Sun SN, Hu XL, Huang YX, Shi W, Jian D. 2017; G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J Steroid Biochem Mol Biol. 165(Pt B):236–246. DOI: 10.1016/j.jsbmb.2016.06.012. PMID: 27378491.
Article64. Seidlová-Wuttke D, Becker T, Christoffel V, Jarry H, Wuttke W. 2003; Silymarin is a selective estrogen receptor beta (ERbeta) agonist and has estrogenic effects in the metaphysis of the femur but no or antiestrogenic effects in the uterus of ovariectomized (ovx) rats. J Steroid Biochem Mol Biol. 86:179–188. DOI: 10.1016/S0960-0760(03)00270-X. PMID: 14568570.
Article65. Choo SJ, Ryoo IJ, Kim YH, Xu GH, Kim WG, Kim KH, Moon SJ, Son ED, Bae K, Yoo ID. 2009; Silymarin inhibits melanin synthesis in melanocyte cells. J Pharm Pharmacol. 61:663–667. DOI: 10.1211/jpp.61.05.0016. PMID: 19406006.
Article66. Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. 2005; MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res. 571:133–152. DOI: 10.1016/j.mrfmmm.2004.09.014. PMID: 15748644.
Article67. Chung S, Lim GJ, Lee JY. 2019; Quantitative analysis of melanin content in a three-dimensional melanoma cell culture. Sci Rep. 9:780. DOI: 10.1038/s41598-018-37055-y. PMID: 30692593. PMCID: PMC6349835.
Article68. Abdel-Malek ZA, Kadekaro AL, Swope VB. 2010; Stepping up melanocytes to the challenge of UV exposure. Pigment Cell Melanoma Res. 23:171–186. DOI: 10.1111/j.1755-148X.2010.00679.x. PMID: 20128873.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Baicalein Inhibits αα-Melanocyte-stimulating Hormonestimulated Melanogenesis via p38 Mitogen-activated Protein Kinase Pathway in B16F10 Mouse Melanoma Cells
- Ethyl linoleate inhibits α-MSH-induced melanogenesis through Akt/GSK3β/β-catenin signal pathway
- Scopoletin from Cirsium setidens Increases Melanin Synthesis via CREB Phosphorylation in B16F10 Cells
- Multi-facet expressions of adenylate cyclase isoforms in B16-F10 melanoma cells differentiated by forskolin treatment
- Dipeptides Inhibit Melanin Synthesis in Mel-Ab Cells through Down-Regulation of Tyrosinase