Cancer Res Treat.
2023 Jul;55(3):1011-1022. 10.4143/crt.2022.1407.
Clinical Significance of bZIP In-Frame CEBPA-Mutated Normal Karyotype Acute Myeloid Leukemia
- Affiliations
-
- 1Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 2The Donnelly Centre for Cellular and Biomolecular Research, University of Toronto, Toronto, Canada
- 3Department of Computer Science, University of Toronto, Toronto, Canada
- 4Genomic Research Center for Hematopoietic Diseases, Chonnam National University Hwasun Hospital, Hwasun, Korea
- 5Division of Hematology-Oncology, Samsung Medical Center, Seoul, Korea
- 6Department of Hematology, Cancer Research Institute, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 7Department of Hematology-Oncology, Kyungpook National University Hospital, Daegu, Korea
- 8Department of Hematology-Oncology, Soon Chun Hyang University Hospital, Seoul, Korea
- 9Department of Hematology-Oncology, Dong-A University College of Medicine, Busan, Korea
- 10Department of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University of Toronto, Toronto, Canada
- KMID: 2544181
- DOI: http://doi.org/10.4143/crt.2022.1407
Abstract
- Purpose
We evaluated the characteristics of CCAAT/enhancer-binding protein α (CEBPA) mutations and the significance of a basic leucine zipper in-frame mutation (bZIPin-f) of CEBPA in patients with acute myeloid leukemia with a normal karyotype.
Materials and Methods
Based on updated knowledge of CEBPA mutations, we conducted next-generation sequencing analyses in a previously established real-world cohort.
Results
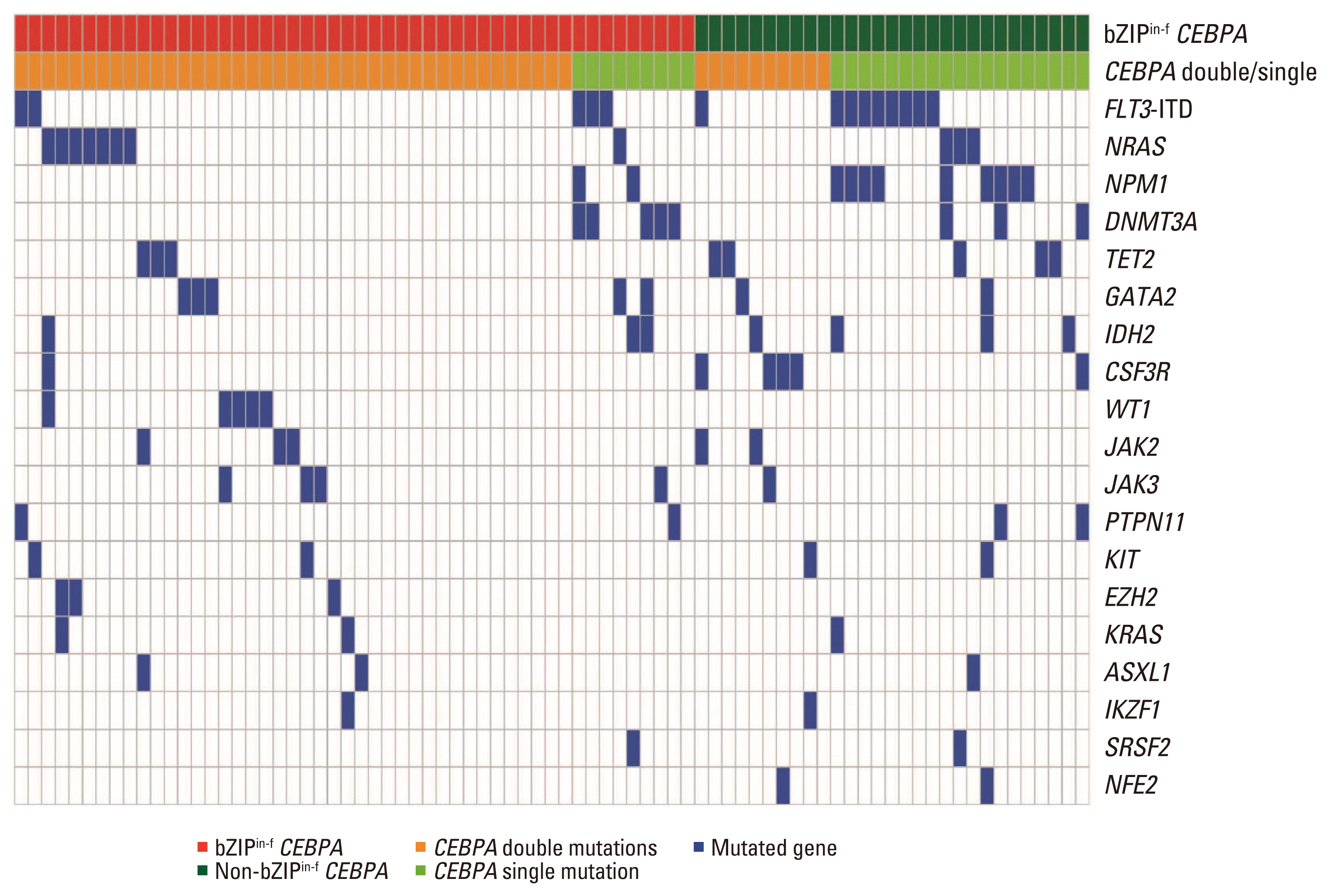
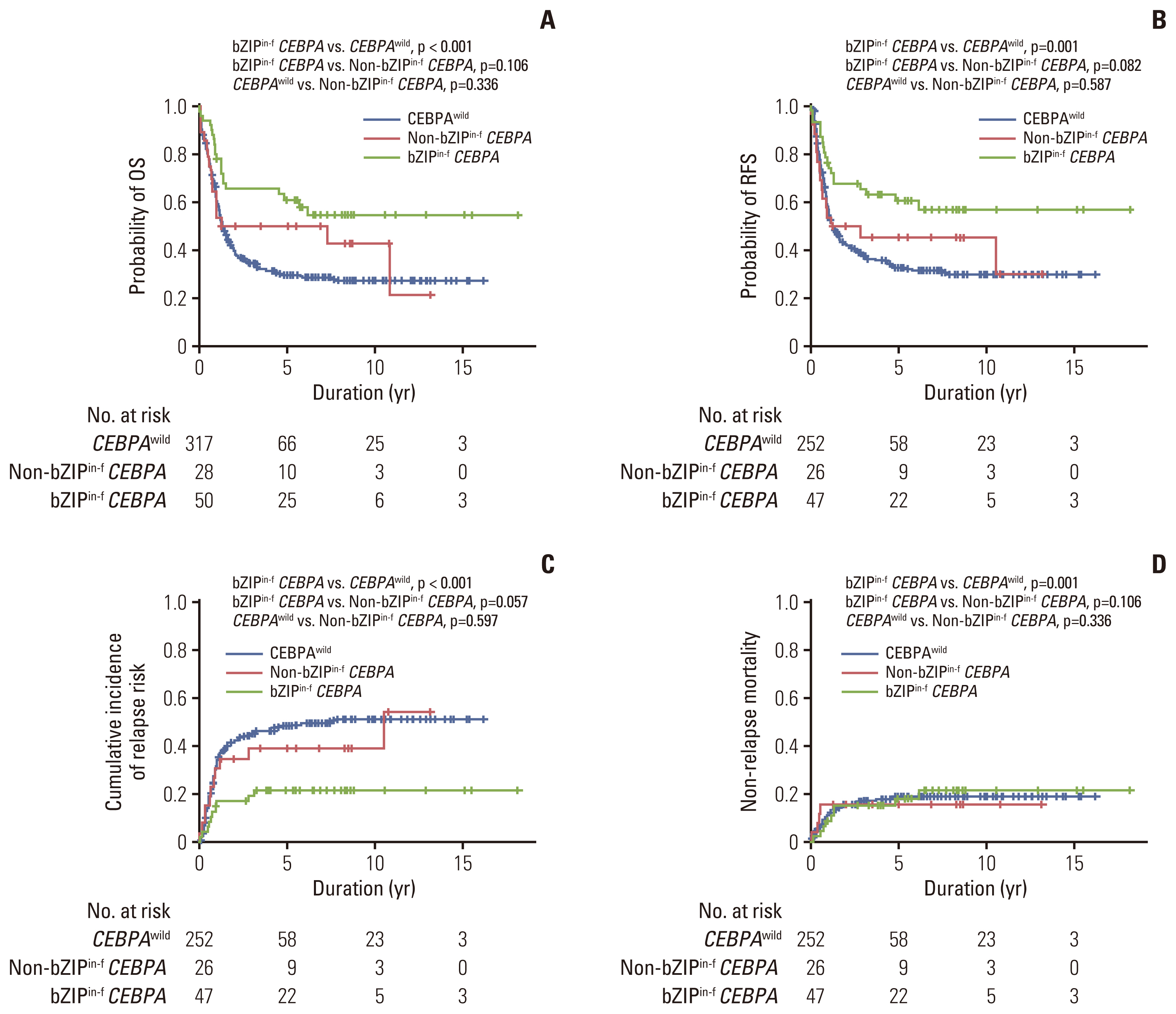
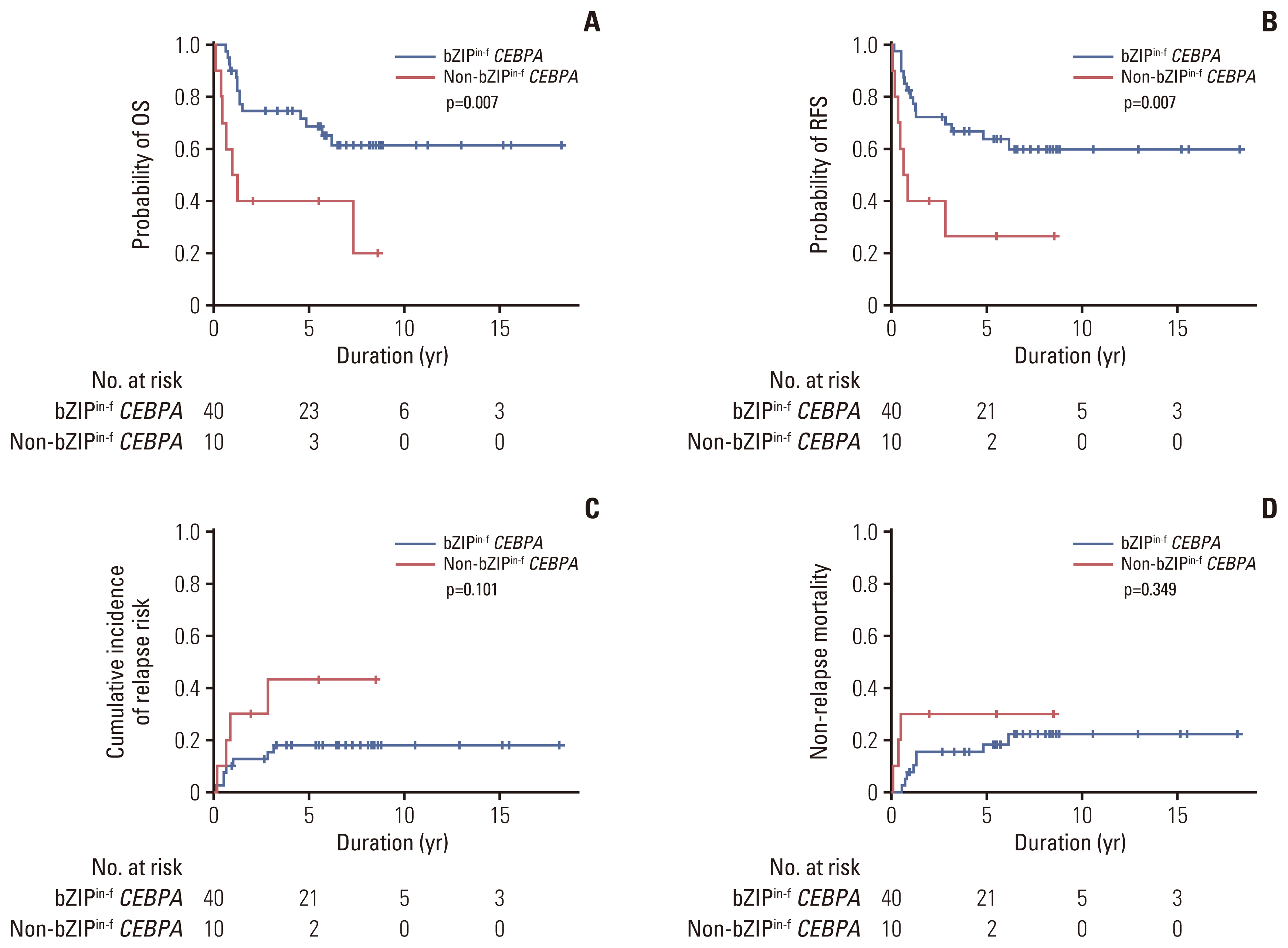
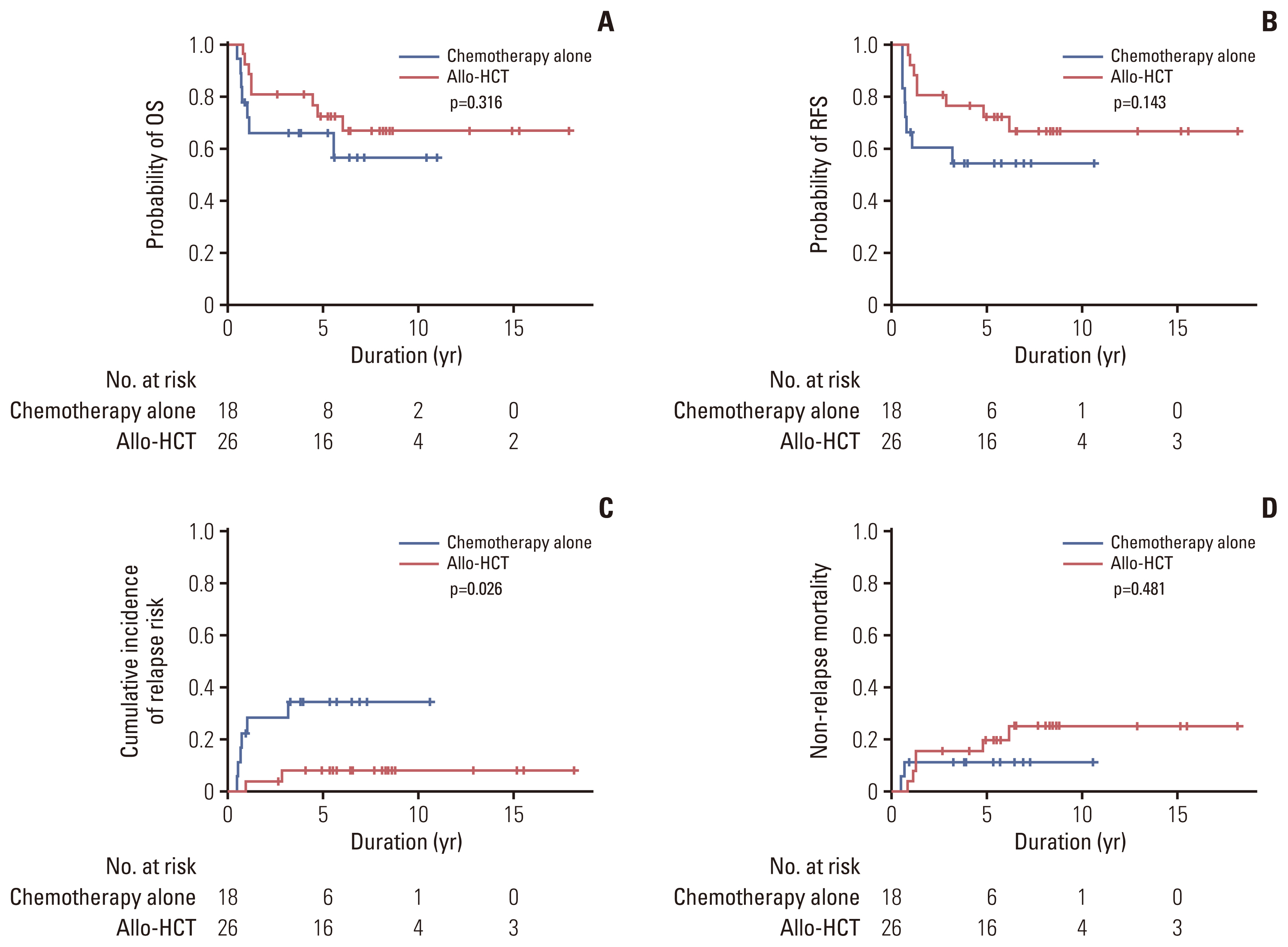
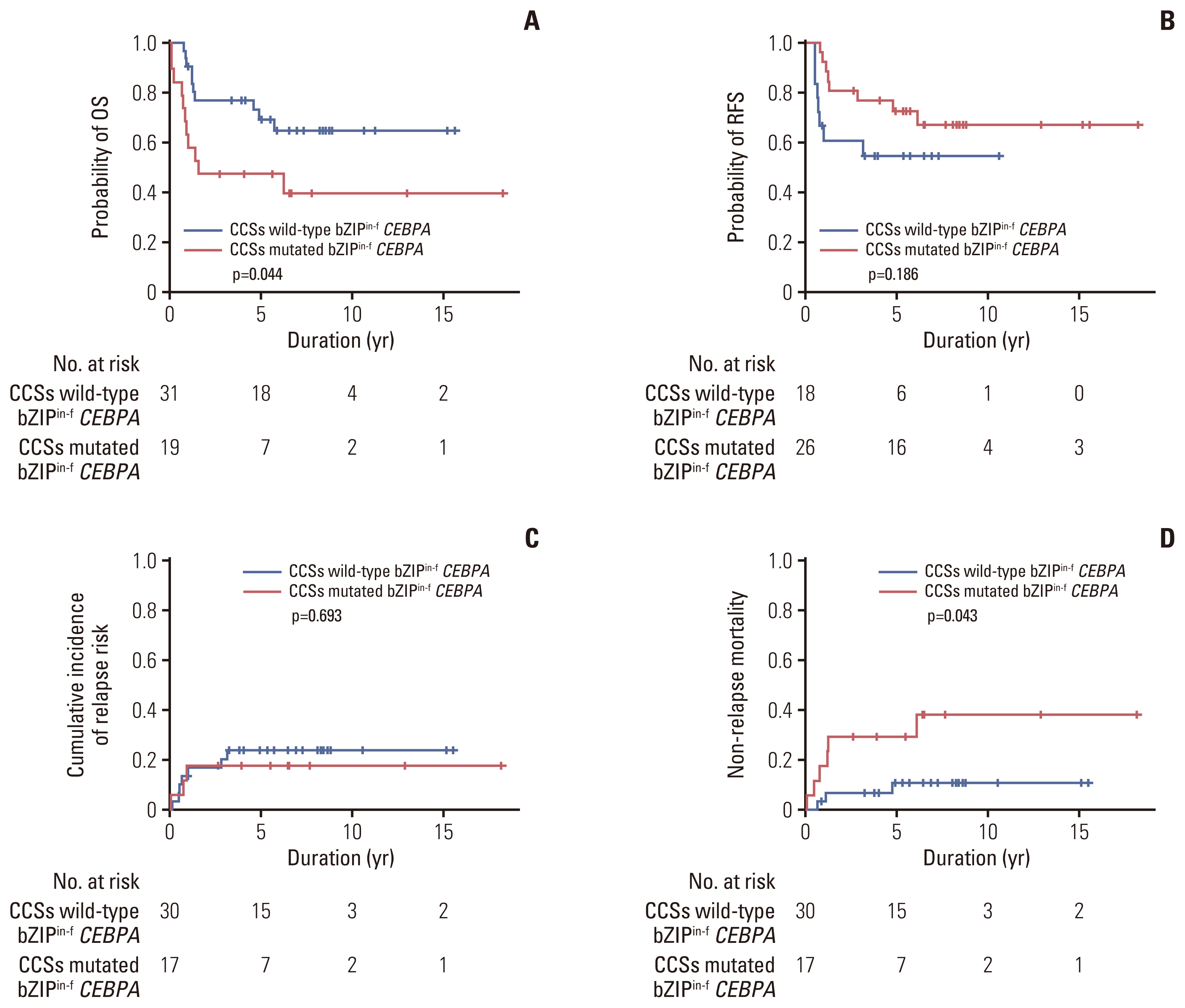
Among 78 of a total of 395 patients (19.7%), 50 had bZIPin-f CEBPA, and 28 had non-bZIPin-f CEBPA. In the multivariate analysis, patients with NPM1mut, those with bZIPin-f CEBPA, and those who underwent allogeneic hematopoietic cell transplantation (allo-HCT) had favorable overall survival (OS), but FLT3-ITDmut was a poor prognostic indicator. For relapse-free survival (RFS) and cumulative incidence of relapse, bZIPin-f CEBPA, and allo-HCT were associated with favorable outcomes; FLT3-ITDpos was associated with worse outcomes. In the CEBPA double-mutated group (CEBPAdm), bZIPin-f CEBPA was associated with superior outcomes in terms of OS (p=0.007) and RFS (p=0.007) compared with non-bZIPin-f CEBPA. Of 50 patients with bZIPin-f CEBPA, 36 patients had at least one mutation. When grouped by the presence of mutations in chromatic/DNA modifiers (C), cohesion complex (C), and splicing genes (S) (CCS mutations), CCS-mutated bZIPin-f CEBPA was associated with poor OS (p=0.044; hazard ratio [HR], 2.419) and a trend in inferior RFS (p=0.186; HR, 1.838).
Conclusion
Only bZIPin-f CEBPA was associated with favorable outcomes in patients with CEBPAdm. However, some mutations accompanying bZIPin-f CEBPA showed inferior OS; thus, further studies with larger numbers of patients are required for clear conclusions of the significance of bZIPin-f CEBPA.
Figure
Reference
-
References
1. Koschmieder S, Halmos B, Levantini E, Tenen DG. Dysregulation of the C/EBPalpha differentiation pathway in human cancer. J Clin Oncol. 2009; 27:619–28.2. Martelli MP, Sportoletti P, Tiacci E, Martelli MF, Falini B. Mutational landscape of AML with normal cytogenetics: biological and clinical implications. Blood Rev. 2013; 27:13–22.
Article3. Pabst T, Mueller BU, Zhang P, Radomska HS, Narravula S, Schnittger S, et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPalpha), in acute myeloid leukemia. Nat Genet. 2001; 27:263–70.4. Green CL, Koo KK, Hills RK, Burnett AK, Linch DC, Gale RE. Prognostic significance of CEBPA mutations in a large cohort of younger adult patients with acute myeloid leukemia: impact of double CEBPA mutations and the interaction with FLT3 and NPM1 mutations. J Clin Oncol. 2010; 28:2739–47.5. Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood. 2013; 122:1576–82.
Article6. Fasan A, Haferlach C, Alpermann T, Jeromin S, Grossmann V, Eder C, et al. The role of different genetic subtypes of CEBPA mutated AML. Leukemia. 2014; 28:794–803.
Article7. Park SH, Chi HS, Cho YU, Jang S, Park CJ. CEBPA single mutation can be a possible favorable prognostic indicator in NPM1 and FLT3-ITD wild-type acute myeloid leukemia patients with intermediate cytogenetic risk. Leuk Res. 2013; 37:1488–94.
Article8. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer;2008.9. Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020; 55:S1–4.
Article10. Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008; 358:1909–18.
Article11. Wouters BJ, Lowenberg B, Erpelinck-Verschueren CA, van Putten WL, Valk PJ, Delwel R. Double CEBPA mutations, but not single CEBPA mutations, define a subgroup of acute myeloid leukemia with a distinctive gene expression profile that is uniquely associated with a favorable outcome. Blood. 2009; 113:3088–91.
Article12. Li HY, Deng DH, Huang Y, Ye FH, Huang LL, Xiao Q, et al. Favorable prognosis of biallelic CEBPA gene mutations in acute myeloid leukemia patients: a meta-analysis. Eur J Haematol. 2015; 94:439–48.
Article13. Ahn JS, Kim JY, Kim HJ, Kim YK, Lee SS, Jung SH, et al. Normal karyotype acute myeloid leukemia patients with CEBPA double mutation have a favorable prognosis but no survival benefit from allogeneic stem cell transplant. Ann Hematol. 2016; 95:301–10.14. Tarlock K, Lamble AJ, Wang YC, Gerbing RB, Ries RE, Loken MR, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the Children’s Oncology Group. Blood. 2021; 138:1137–47.15. Wakita S, Sakaguchi M, Oh I, Kako S, Toya T, Najima Y, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022; 6:238–47.16. Taube F, Georgi JA, Kramer M, Stasik S, Middeke JM, Rollig C, et al. CEBPA mutations in 4708 patients with acute myeloid leukemia: differential impact of bZIP and TAD mutations on outcome. Blood. 2022; 139:87–103.
Article17. Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022; 140:1345–77.18. Ahn JS, Kim HJ, Kim YK, Lee SS, Ahn SY, Jung SH, et al. Assessment of a new genomic classification system in acute myeloid leukemia with a normal karyotype. Oncotarget. 2018; 9:4961–8.
Article19. Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129:424–47.
Article20. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48:452–8.
Article21. Su L, Shi YY, Liu ZY, Gao SJ. Acute myeloid leukemia with CEBPA mutations: current progress and future directions. Front Oncol. 2022; 12:806137.
Article22. Su L, Tan Y, Lin H, Liu X, Yu L, Yang Y, et al. Mutational spectrum of acute myeloid leukemia patients with double CEBPA mutations based on next-generation sequencing and its prognostic significance. Oncotarget. 2018; 9:24970–9.
Article23. Konstandin NP, Pastore F, Herold T, Dufour A, Rothenberg-Thurley M, Hinrichsen T, et al. Genetic heterogeneity of cytogenetically normal AML with mutations of CEBPA. Blood Adv. 2018; 2:2724–31.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Novel Mutations in CEBPA in Korean Patients with Acute Myeloid Leukemia with a Normal Karyotype
- Reclassification of Acute Myeloid Leukemia According to the 2016 WHO Classification
- Prognostic Significance of FLT3 Internal Tandem Duplication in Acute Myeloid Leukemia with Normal Karyotype
- Double Minutes Containing C-MYC oncogene with a Normal Karyotype in Acute Myelogenous Leukemia: A Case Report
- FLT3 mutations in acute myeloid leukemia: a review focusing on clinically applicable drugs