Reclassification of Acute Myeloid Leukemia According to the 2016 WHO Classification
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. microkim@catholic.ac.kr, yonggoo@catholic.ac.kr
- 2Department of Hematology, Seoul St. Mary's Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Leukemia Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Catholic Genetic Laboratory Center, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2431609
- DOI: http://doi.org/10.3343/alm.2019.39.3.311
Abstract
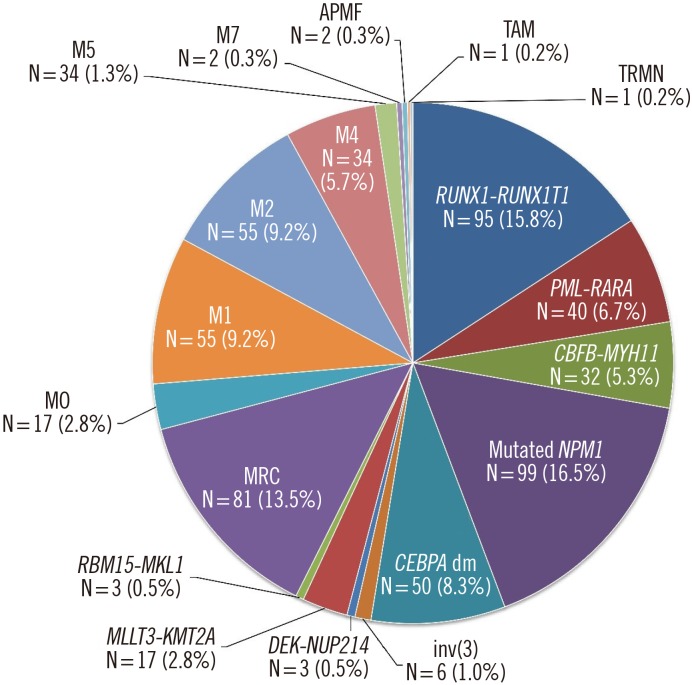
- We reviewed our leukemia database to reclassify 610 patients previously diagnosed as having acute myeloid leukemia (AML) according to the updated 2016 WHO classification. Nine patients were categorized as having myelodysplastic syndrome and myeloid neoplasms with germline predisposition. AML with recurrent genetic abnormalities accounted for 57.4% (345/601) of the patients under the 2016 WHO classification. AML with mutated NPM1 was the most common form (16.5%), with the majority associated with monocytic differentiation (63.6%). AML with double CEBPA mutations accounted for 8.3% of these cases, and the majority were previously diagnosed as AML with/without maturation (78.0%). These newly classified mutations were mutually exclusive without overlapping with other forms of AML with recurrent genetic abnormalities. AML with mutated NPM1 and AML with myelodysplasia-related changes comprised the oldest patients, whereas AML with RUNX1-RUNX1T1 included the youngest patients. The leukocyte count was highest in AML with mutated NPM1, and the percentage of peripheral blood blasts was the highest in AML with double CEBPA mutations. Our results indicate that implementation of the 2016 WHO classification of AML would not pose major difficulties in clinical practice. Hematopathologists should review and prepare genetic tests for the new classification, according to their clinical laboratory conditions.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Changing the frequency and spectra of chromosomal aberrations in Korean patients with acute leukemia in a tertiary care hospital
Je-Hyun Park, Min-Gu Kang, Hye-Ran Kim, Young-Eun Lee, Jun Hyung Lee, Hyun-Jung Choi, Jong-Hee Shin, Myung-Geun Shin
Blood Res. 2020;55(4):225-245. doi: 10.5045/br.2020.2020255.급성골수성백혈병의 개정된 세계보건기구 기준에 의한 대규모 환자군의 분류: 재분류된 사례의 빈도 및 특성
Sihwan Kim, Min-Young Lee, Young-Uk Cho, Sang-Hyun Hwang, Seongsoo Jang, Eul-Ju Seo, Chan-Jeoung Park
Lab Med Online. 2021;11(2):115-123. doi: 10.47429/lmo.2021.11.2.115.Real-World Treatment Patterns and Clinical Outcomes in Korean Patients With AML Ineligible for First-Line Intensive Chemotherapy: A Subanalysis of the CURRENT Study, a Non-Interventional, Retrospective Chart Review
Soo-Mee Bang, Ka-Won Kang, Ik-Chan Song, Cynthia Llamas, Yinghui Duan, Ji-Young Jeong, Je-Hwan Lee
J Korean Med Sci. 2023;38(44):e345. doi: 10.3346/jkms.2023.38.e345.
Reference
-
1. Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114:937–951. PMID: 19357394.2. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. PMID: 27069254.3. McGowan-Jordan J, Simons A, Schmid M. ISCN 2016: an international system for human cytogenomic nomenclature. Basel, New York: Karger;2016. p. 7–99.4. Döhner K, Schlenk RF, Habdank M, Scholl C, Rücker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005; 106:3740–3746. PMID: 16051734.5. Falini B, Sportoletti P, Martelli MP. Acute myeloid leukemia with mutated NPM1: diagnosis, prognosis and therapeutic perspectives. Curr Opin Oncol. 2009; 21:573–581. PMID: 19770764.6. Haferlach C, Mecucci C, Schnittger S, Kohlmann A, Mancini M, Cuneo A, et al. AML with mutated NPM1 carrying a normal or aberrant karyotype show overlapping biologic, pathologic, immunophenotypic, and prognostic features. Blood. 2009; 114:3024–3032. PMID: 19429869.7. Lin LI, Chen CY, Lin DT, Tsay W, Tang JL, Yeh YC, et al. Characterization of CEBPA mutations in acute myeloid leukemia: most patients with CEBPA mutations have biallelic mutations and show a distinct immunophenotype of the leukemic cells. Clin Cancer Res. 2005; 11:1372–1379. PMID: 15746035.8. Ho PA, Alonzo TA, Gerbing RB, Pollard J, Stirewalt DL, Hurwitz C, et al. Prevalence and prognostic implications of CEBPA mutations in pediatric acute myeloid leukemia (AML): a report from the Children's Oncology Group. Blood. 2009; 113:6558–6566. PMID: 19304957.9. Li HY, Deng DH, Huang Y, Ye FH, Huang LL, Xiao Q, et al. Favorable prognosis of biallelic CEBPA gene mutations in acute myeloid leukemia patients: a meta-analysis. Eur J Haematol. 2015; 94:439–448. PMID: 25227715.10. Grossmann V, Haferlach C, Nadarajah N, Fasan A, Weissmann S, Roller A, et al. CEBPA double-mutated acute myeloid leukaemia harbours concomitant molecular mutations in 76.8% of cases with TET2 and GATA2 alterations impacting prognosis. Br J Haematol. 2013; 161:649–658. PMID: 23521373.11. Dufour A, Schneider F, Metzeler KH, Hoster E, Schneider S, Zellmeier E, et al. Acute myeloid leukemia with biallelic CEBPA gene mutations and normal karyotype represents a distinct genetic entity associated with a favorable clinical outcome. J Clin Oncol. 2010; 28:570–577. PMID: 20038735.12. Salipante SJ, Fromm JR, Shendure J, Wood BL, Wu D. Detection of minimal residual disease in NPM1-mutated acute myeloid leukemia by next-generation sequencing. Mod Pathol. 2014; 27:1438–1446. PMID: 24743218.13. Behdad A, Weigelin HC, Elenitoba-Johnson KS, Betz BL. A clinical grade sequencing-based assay for CEBPA mutation testing: report of a large series of myeloid neoplasms. J Mol Diagn. 2015; 17:76–84. PMID: 25468431.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Classification of acute myeloid leukemia
- Acute Myeloid Leukemia with Intracardiac Thrombus Presenting as Acute Limb Ischemia
- A Pediatric Case of Acute Myeloid Leukemia with t(3;5)(q25;q34)
- Myeloid Sarcoma of Peritoneum in Acute Myeloid Leukemia Patient with Inversion of Chromosome 16
- A Case of Myeloid Sarcoma Preceding the Diagnosis of Acute Myeloid Leukemia