Int J Stem Cells.
2023 Feb;16(1):66-77. 10.15283/ijsc21146.
Efficacy and Safety of Human Bone Marrow-Derived Mesenchymal Stem Cells according to Injection Route and Dose in a Chronic Kidney Disease Rat Model
- Affiliations
-
- 1Department of Urology, Gangneung Asan Medical Center, University of Ulsan College of Medicine, Gangneung, Korea
- 2Department of Pharmaceutical Engineering, College of Medical Sciences and Department of Medical Sciences, General Graduate School, Soon Chun Hyang University, Asan, Korea
- 3Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea
- 4Department of Urology, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 5Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea 6 Pharmicell Co. Ltd., Seongnam, Korea
- KMID: 2539622
- DOI: http://doi.org/10.15283/ijsc21146
Abstract
- Background and Objectives
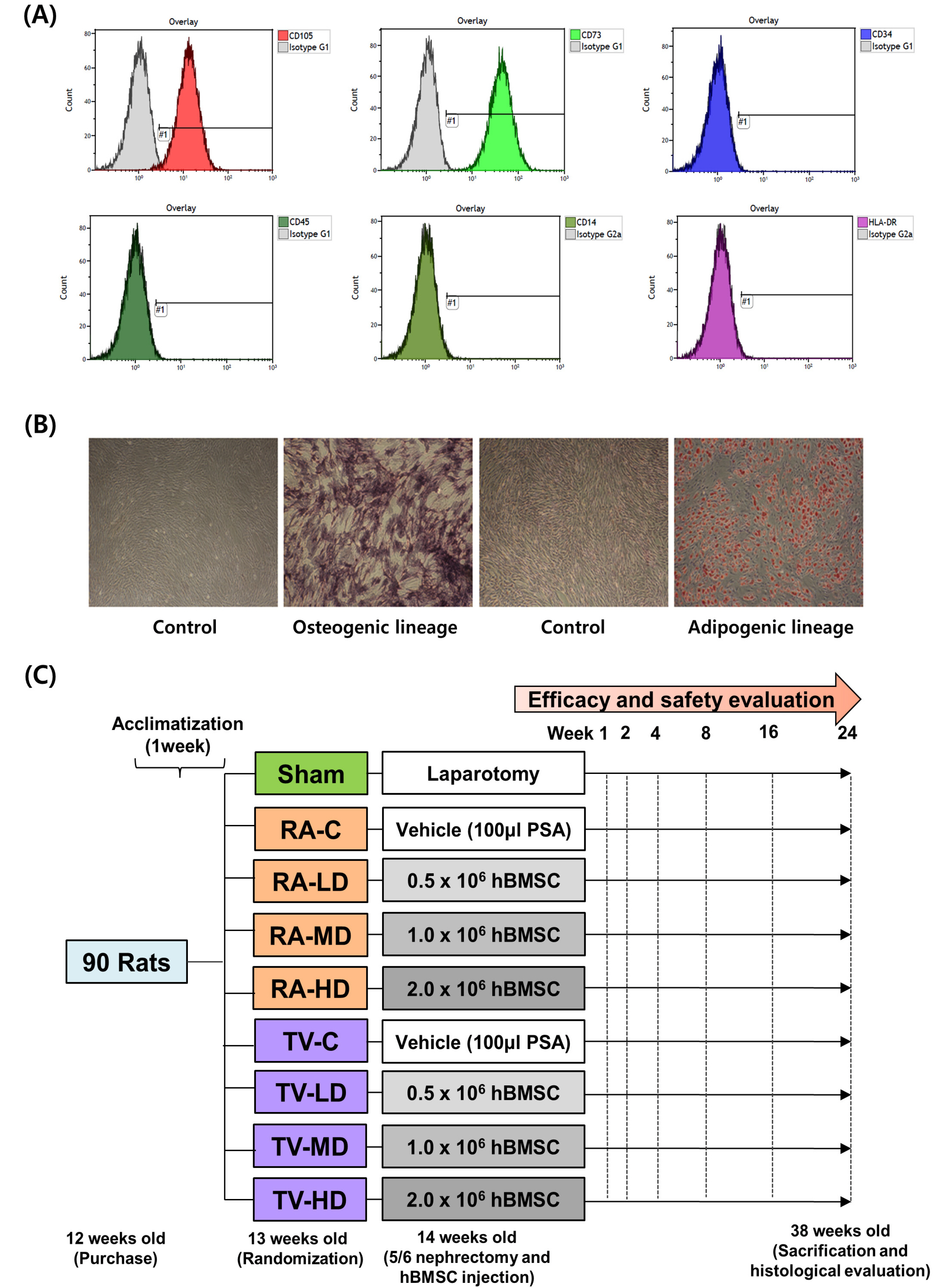
We compared the efficacy and safety of human bone marrow-derived mesenchymal stem cells (hBMSC), delivered at different doses and via different injection routes in an animal model of chronic kidney disease.
Methods and Results
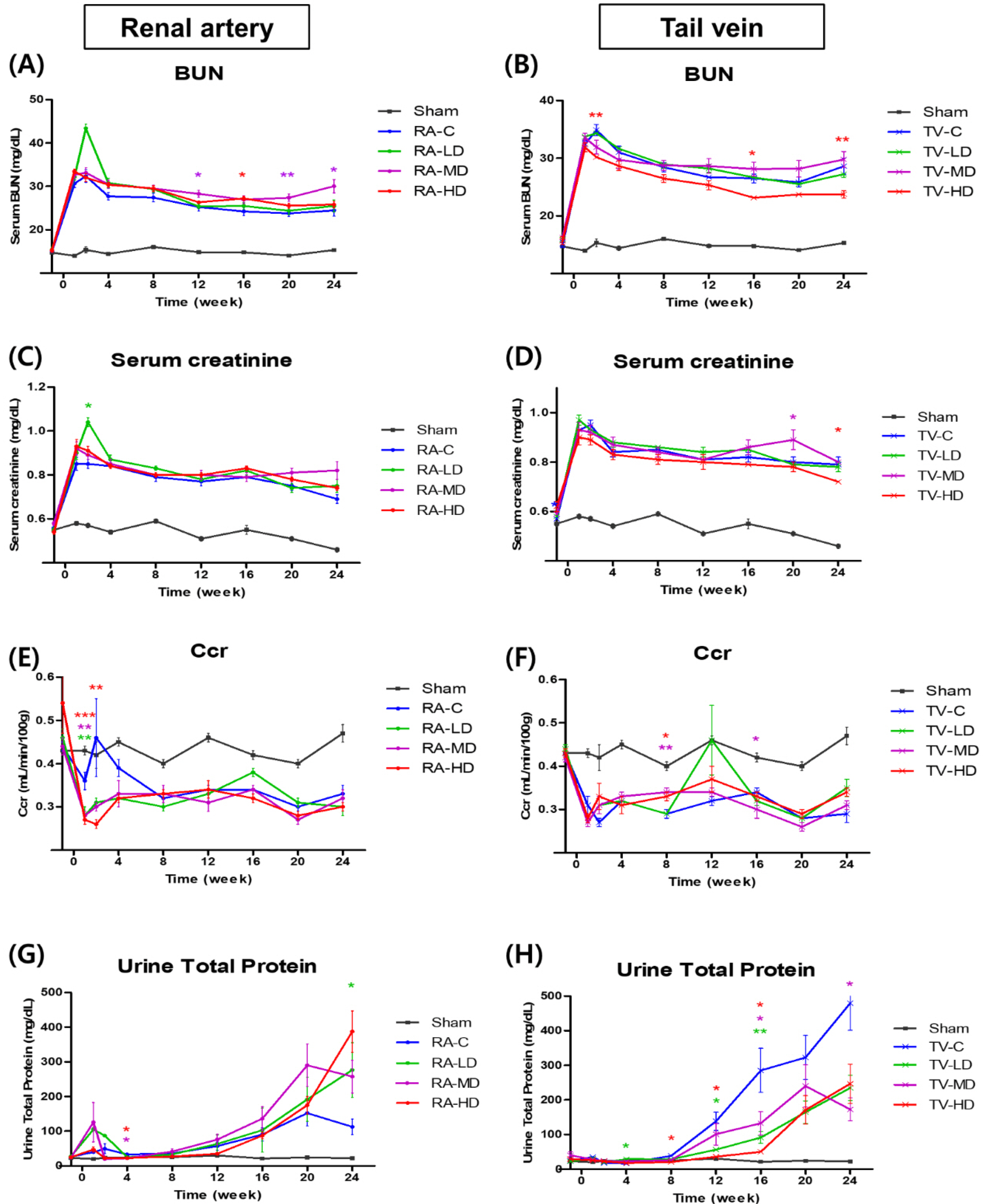
A total of ninety 12-week-old rats underwent 5/6 nephrectomy and randomized among nine groups: sham, renal artery control (RA-C), tail vein control (TV-C), renal artery low dose (RA-LD) (0.5×10 6 cells), renal artery moderate dose (RA-MD) (1.0×10 6 cells), renal artery high dose (RA-HD) (2.0×10 6 cells), tail vein low dose (TV-LD) (0.5×10 6 cells), tail vein moderate dose (TV-MD) (1.0×10 6 cells), and tail vein high dose (TV-HD) (2.0×10 6 cells). Renal function and mortality of rats were evaluated after hBMSC injection. Serum blood urea nitrogen was significantly lower in the TV-HD group at 2 weeks (p<0.01), 16 weeks (p<0.05), and 24 weeks (p<0.01) than in the TV-C group, as determined by one-way ANOVA. Serum creatinine was significantly lower in the TV-HD group at 24 weeks (p<0.05). At 8 weeks, creatinine clearance was significantly higher in the TV-MD and TV-HD groups (p<0.01, p<0.05) than in the TV-C group. In the safety evaluation, we observed no significant difference among the groups.
Conclusions
Our findings confirm the efficacy and safety of high dose (2×10 6 cells) injection of hBMSC via the tail vein.
Figure
Cited by 1 articles
-
Guidelines for Manufacturing and Application of Organoids: Kidney
Hyun Mi Kang, Dong Sung Kim, Yong Kyun Kim, Kunyoo Shin, Sun-Ju Ahn, Cho-Rok Jung
Int J Stem Cells. 2024;17(2):141-146. doi: 10.15283/ijsc24040.
Reference
-
References
1. Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. 1974; Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. cloning in vitro and retransplantation in vivo. Transplantation. 17:331–340. DOI: 10.1097/00007890-197404000-00001. PMID: 4150881.2. Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. 2018; Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 7:651–663. DOI: 10.1002/sctm.18-0024. PMID: 30070053. PMCID: PMC6127224. PMID: 7559fd62178645e6b65db7694242911c.3. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Kim AS, Johnson JN, Bates D, Poggio G, Case C, McGrogan M, Yankee EW, Schwartz NE. 2018; Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg. 131:1462–1472. DOI: 10.3171/2018.5.JNS173147. PMID: 30497166.4. Reinders ME, Dreyer GJ, Bank JR, Roelofs H, Heidt S, Roelen DL, Zandvliet ML, Huurman VA, Fibbe WE, van Kooten C, Claas FH, Rabelink TJ, de Fijter JW. 2015; Safety of allogeneic bone marrow derived mesenchymal stromal cell therapy in renal transplant recipients: the neptune study. J Transl Med. 13:344. DOI: 10.1186/s12967-015-0700-0. PMID: 26537851. PMCID: PMC4632480.5. Cai J, Yu X, Xu R, Fang Y, Qian X, Liu S, Teng J, Ding X. 2014; Maximum efficacy of mesenchymal stem cells in rat model of renal ischemia-reperfusion injury: renal artery administration with optimal numbers. PLoS One. 9:e92347. Erratum in: PLoS One 2014;9:e101336. DOI: 10.1371/journal.pone.0092347. PMID: 24637784. PMCID: PMC3956922. PMID: ce0c5531399d45dfa494850e9b457c67.6. National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals. 2011. Guide for the care and use of laboratory animals. 8th ed. National Academies Press;Washington, D.C.:7. Hamed GM, Morsy WE, Hamid MSA, Hassan AAEM, Zahra FAA. 2019; Effect of bone marrow-derived mesenchymal stem cells on ischaemic-reperfused hearts in adult rats with established chronic kidney disease. Int J Stem Cells. 12:304–314. DOI: 10.15283/ijsc18114. PMID: 31022998. PMCID: PMC6657945.8. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. 2009; Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 106:14022–14027. DOI: 10.1073/pnas.0903201106. PMID: 19666564. PMCID: PMC2729013.9. Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. 2015; Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 8:281–293. DOI: 10.1242/dmm.017699. PMID: 25633980. PMCID: PMC4348565.10. Uccelli A, de Rosbo NK. 2015; The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 1351:114–126. DOI: 10.1111/nyas.12815. PMID: 26152292.11. Saldanha-Araujo F, Ferreira FI, Palma PV, Araujo AG, Queiroz RH, Covas DT, Zago MA, Panepucci RA. 2011; Mesenchymal stromal cells up-regulate CD39 and increase adenosine production to suppress activated T-lymphocytes. Stem Cell Res. 7:66–74. DOI: 10.1016/j.scr.2011.04.001. PMID: 21546330.12. Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, Bruno S, Amoroso A, Giovarelli M, Porta M, Perin PC, Tetta C, Camussi G, Zanone MM. 2014; Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 57:1664–1673. DOI: 10.1007/s00125-014-3262-4. PMID: 24838680.13. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999; Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.14. Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. 2002; Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 20:530–541. DOI: 10.1634/stemcells.20-6-530. PMID: 12456961.15. Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. 1999; Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 17:171–177. DOI: 10.1007/s007740050081. PMID: 10757676.16. Jang MJ, You D, Park JY, Kim K, Aum J, Lee C, Song G, Shin HC, Suh N, Kim YM, Kim CS. 2018; Hypoxic preconditioned mesenchymal stromal cell therapy in a rat model of renal ischemia-reperfusion injury: development of optimal protocol to potentiate therapeutic efficacy. Int J Stem Cells. 11:157–167. DOI: 10.15283/ijsc18073. PMID: 30497128. PMCID: PMC6285294.17. Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS Jr. 2009; Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18:683–692. DOI: 10.1089/scd.2008.0253. PMID: 19099374. PMCID: PMC3190292.18. Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. 2007; Stem cell transplantation: the lung barrier. Transplant Proc. 39:573–576. DOI: 10.1016/j.transproceed.2006.12.019. PMID: 17362785.19. Wang Y, He J, Pei X, Zhao W. 2013; Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton). 18:201–208. DOI: 10.1111/nep.12018. PMID: 23217027.20. Dahlke MH, Hoogduijn M, Eggenhofer E, Popp FC, Renner P, Slowik P, Rosenauer A, Piso P, Geissler EK, Lange C, Chabannes D, Mazzanti B, Bigenzahn S, Bertolino P, Kunter U, Introna M, Rambaldi A, Capelli C, Perico N, Casiraghi F, Noris M, Gotti E, Seifert M, Saccardi R, Verspaget HW, van Hoek B, Bartholomew A, Wekerle T, Volk HD, Remuzzi G, Deans R, Lazarus H, Schlitt HJ, Baan CC. 2009; Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 88:614–619. DOI: 10.1097/TP.0b013e3181b4425a. PMID: 19741455.21. De Martino M, Zonta S, Rampino T, Gregorini M, Frassoni F, Piotti G, Bedino G, Cobianchi L, Dal Canton A, Dionigi P, Alessiani M. 2010; Mesenchymal stem cells infusion prevents acute cellular rejection in rat kidney transplantation. Transplant Proc. 42:1331–1335. DOI: 10.1016/j.transproceed.2010.03.079. PMID: 20534294.22. Masterson CH, Curley GF, Laffey JG. 2019; Modulating the distribution and fate of exogenously delivered MSCs to enhance therapeutic potential: knowns and unknowns. Intensive Care Med Exp. 7(Suppl 1):41. DOI: 10.1186/s40635-019-0235-4. PMID: 31346794. PMCID: PMC6658643. PMID: c89ebb56557f468abda37693bed42a40.23. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. 2009; Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5:54–63. DOI: 10.1016/j.stem.2009.05.003. PMID: 19570514. PMCID: PMC4154377.24. Zhuo W, Liao L, Fu Y, Xu T, Wu W, Yang S, Tan J. 2013; Efficiency of endovenous versus arterial administration of mesenchymal stem cells for ischemia-reperfusion-induced renal dysfunction in rats. Transplant Proc. 45:503–510. DOI: 10.1016/j.transproceed.2012.07.162. PMID: 23498785.25. Choi SJ, Kim JK, Hwang SD. 2010; Mesenchymal stem cell therapy for chronic renal failure. Expert Opin Biol Ther. 10:1217–1226. DOI: 10.1517/14712598.2010.500284. PMID: 20560782.26. Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N. 2017; Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 8:116. DOI: 10.1186/s13287-017-0557-7. PMID: 28535817. PMCID: PMC5442691.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Survival and Graft versus Host Disease in Murine MHC Mismatched Hematopoietic Stem Cell Transplantation with Co-injection of Mesenchymal Stem Cells
- Bone marrow-derived stem cells contribute to regeneration of the endometrium