Endocrinol Metab.
2022 Dec;37(6):879-890. 10.3803/EnM.2022.1563.
BRAFV600E Mutation Enhances Estrogen-Induced Metastatic Potential of Thyroid Cancer by Regulating the Expression of Estrogen Receptors
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Human Genetics, McGill University, Montreal, QC, Canada
- 3Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 4Division of Surgery, Thyroid Center, Seoul National University Hospital, Seoul, Korea
- 5Department of Surgery, Yanbian University Hospital, Yanji, China
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2537289
- DOI: http://doi.org/10.3803/EnM.2022.1563
Abstract
- Background
Cross-talk between mitogen-activated protein kinase and estrogen has been reported; however, the role of BRAFV600E in the estrogen responsiveness of thyroid cancer is unknown. We elucidated the effect of BRAFV600E on the estrogen-induced increase in metastatic potential in thyroid cancer.
Methods
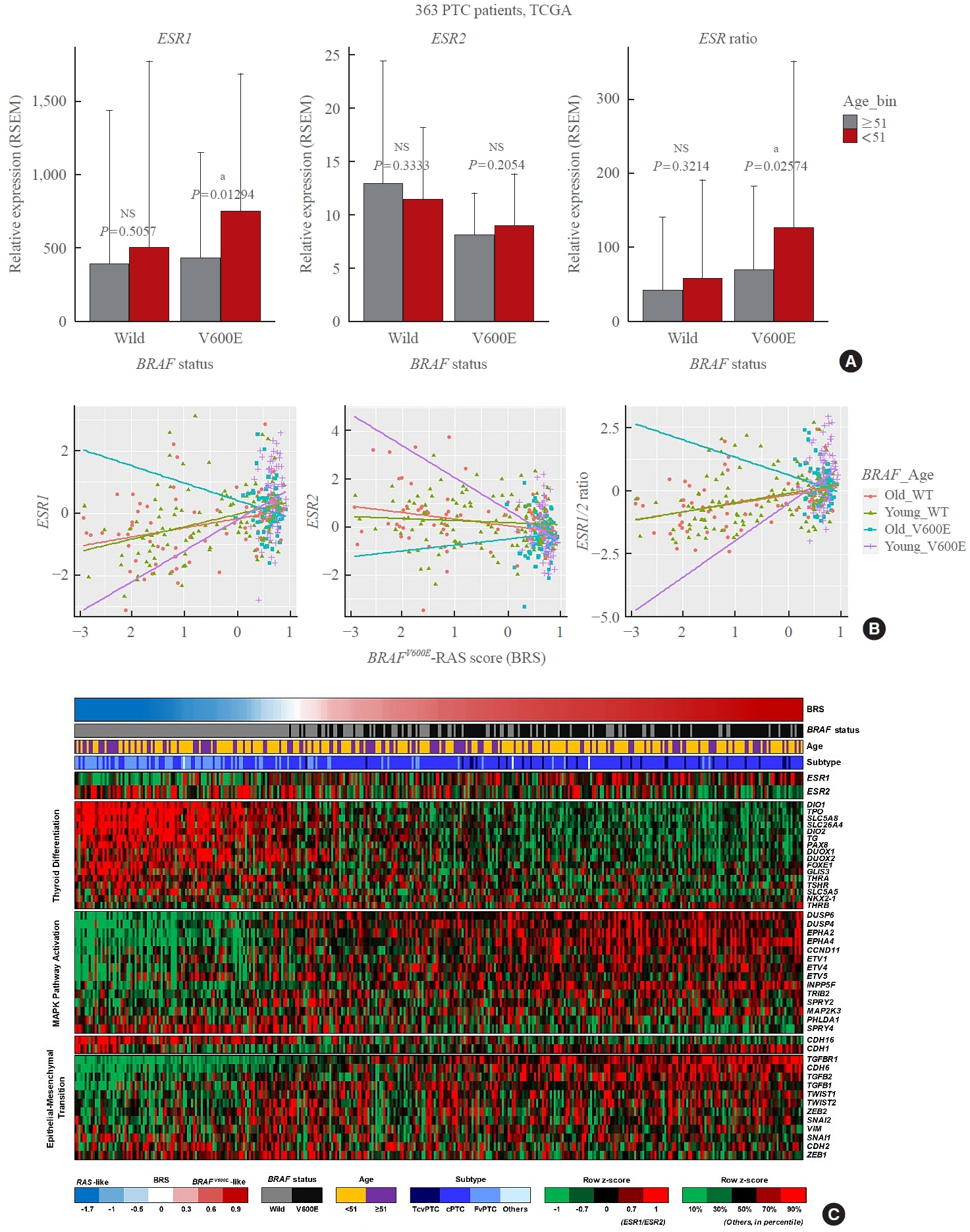
Using a pair of cell lines, human thyroid cell lines which harbor wild type BRAF gene (Nthy/WT) and Nthy/BRAFV600E (Nthy/V600E), the expression of estrogen receptors (ERs) and estrogen-induced metastatic phenotypes were evaluated. Susceptibility to ERα- and ERβ-selective agents was evaluated to confirm differential ER expression. ESR expression was analyzed according to BRAFV600E status and age (≤50 years vs. >50 years) using The Cancer Genome Atlas (TCGA) data.
Results
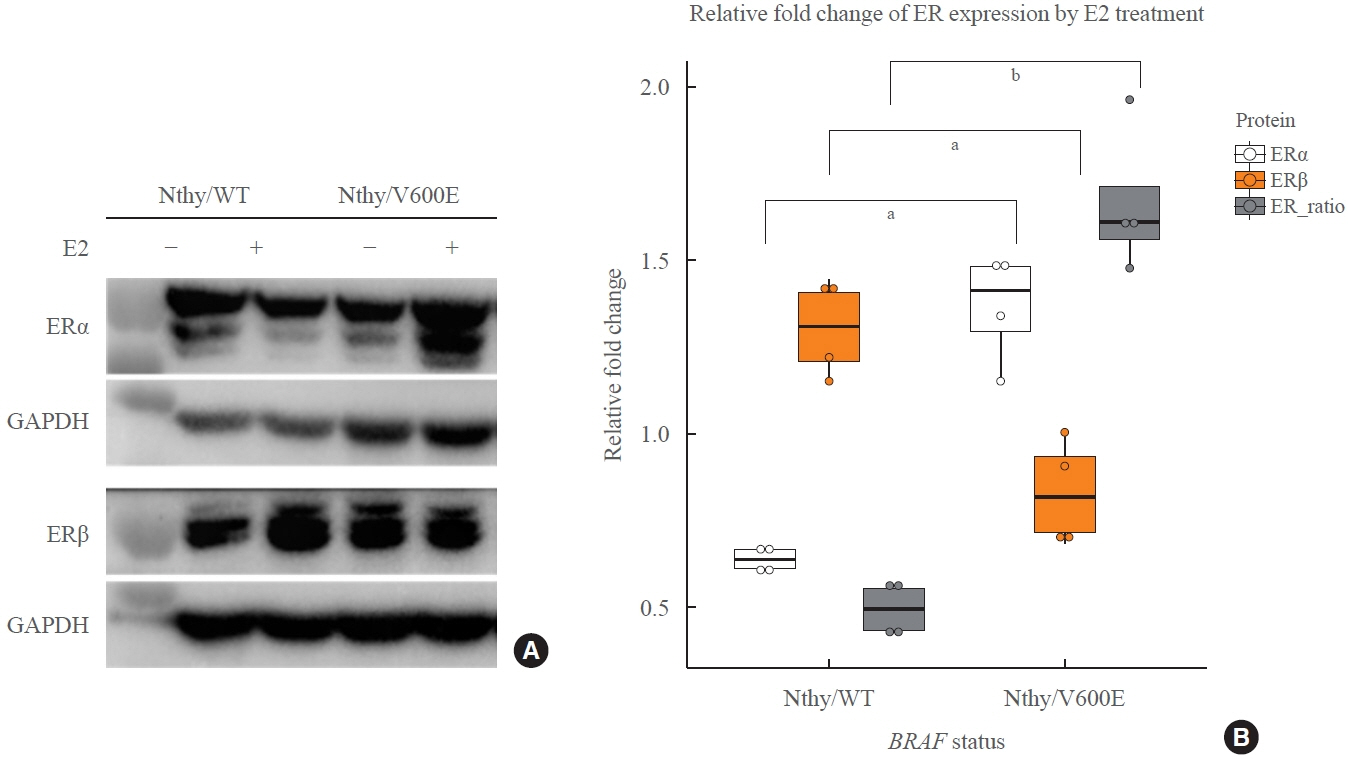
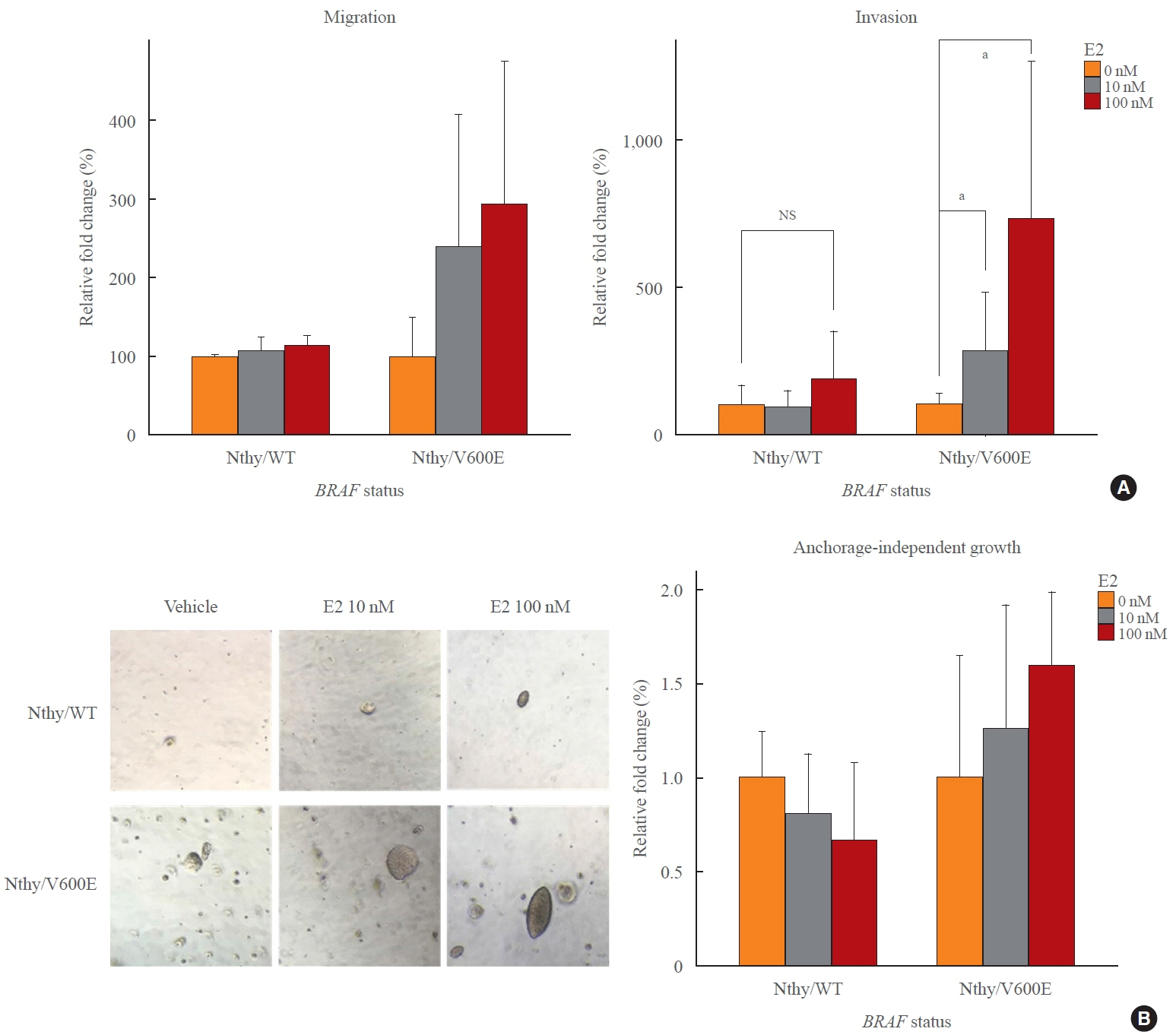
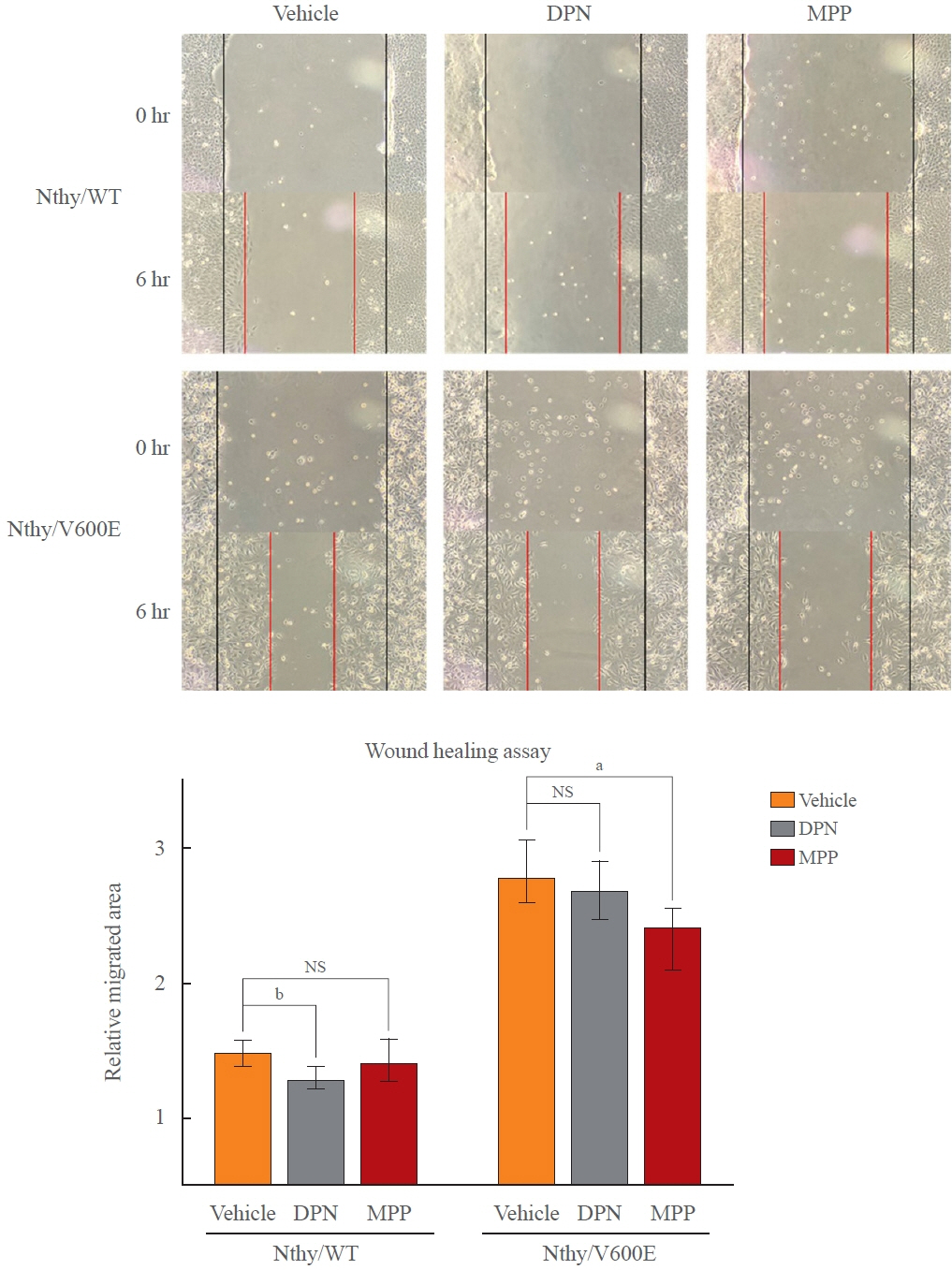
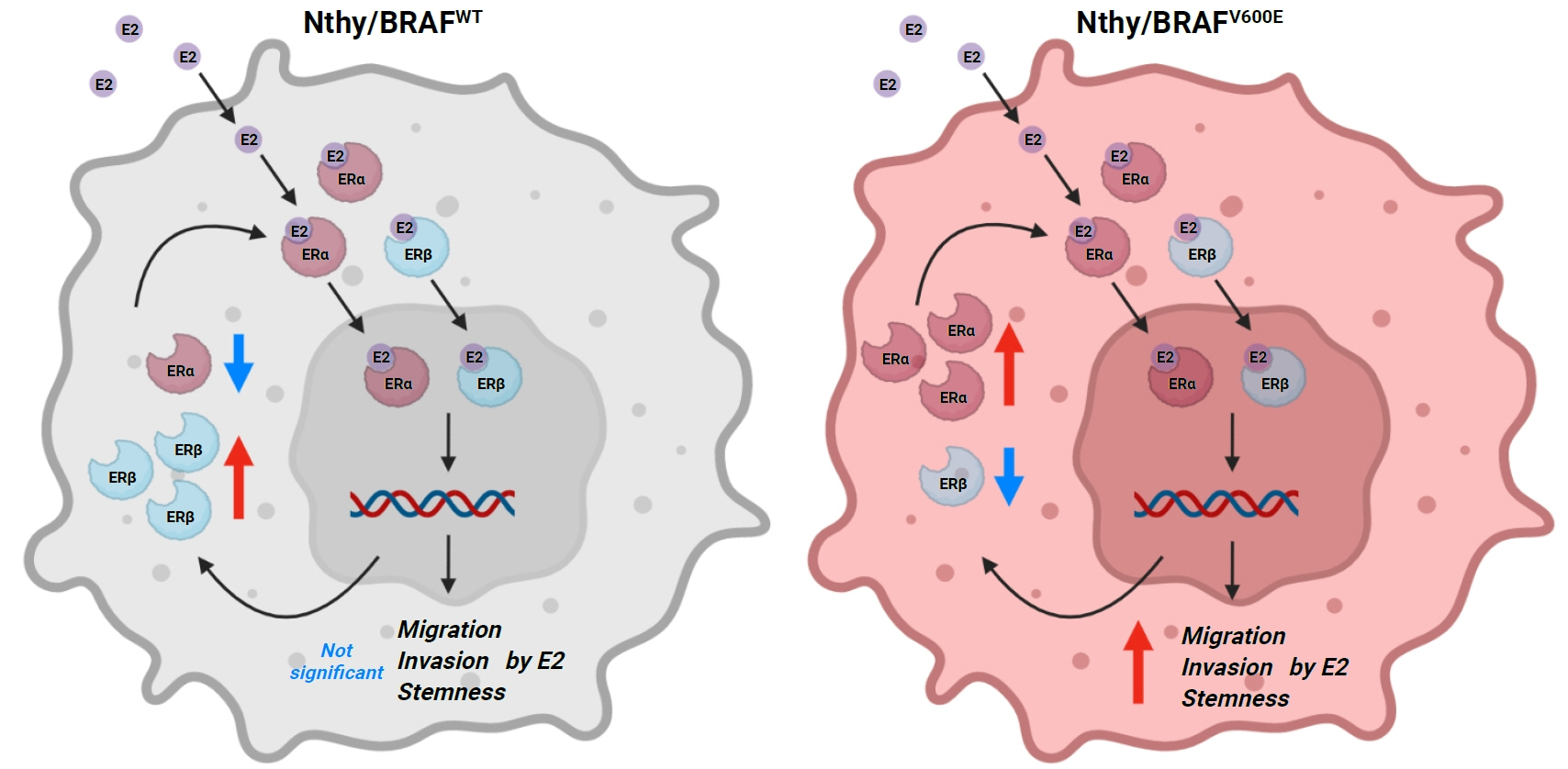
Estradiol increased the ERα/ERβ expression ratio in Nthy/V600E, whereas the decreased ERα/ERβ expression ratio was found in Nthy/WT. BRAFV600E-mutated cell lines showed a higher E2-induced increase in metastatic potential, including migration, invasion, and anchorage-independent growth compared with Nthy/WT. An ERα antagonist significantly inhibited migration in Nthy/V600E cells, whereas an ERβ agonist was more effective in Nthy/WT. In the BRAFV600E group, ESR1/ESR2 ratio was significantly higher in younger age group (≤50 years) compared with older age group (>50 years) by TCGA data analysis.
Conclusion
Our data show that BRAFV600E mutation plays a crucial role in the estrogen responsiveness of thyroid cancer by regulating ER expression. Therefore, BRAFV600E might be used as a biomarker when deciding future hormone therapies based on estrogen signaling in thyroid cancer patients.
Figure
Reference
-
1. National Cancer Institute. SEER Cancer Stat Facts: Thyroid Cancer [Internet]. Bethesda: NIH;2017. [cited 2022 Nov 28]. Available from: https://seer.cancer.gov/statfacts/html/thyro.html.2. Cancer Research UK. Thyroid cancer incidence statistics [Internet]. London: Cancer Research UK;2022. [cited 2022 Nov 28]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancertype/thyroid-cancer/incidence#heading-Two.3. Horn-Ross PL, Canchola AJ, Ma H, Reynolds P, Bernstein L. Hormonal factors and the risk of papillary thyroid cancer in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev. 2011; 20:1751–9.
Article4. Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980-2008. Thyroid. 2013; 23:103–10.
Article5. Yasmeen S, Cress R, Romano PS, Xing G, Berger-Chen S, Danielsen B, et al. Thyroid cancer in pregnancy. Int J Gynaecol Obstet. 2005; 91:15–20.
Article6. Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011; 38:425–40.
Article7. Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996; 81:1495–501.
Article8. Rahbari R, Zhang L, Kebebew E. Thyroid cancer gender disparity. Future Oncol. 2010; 6:1771–9.
Article9. Derwahl M, Nicula D. Estrogen and its role in thyroid cancer. Endocr Relat Cancer. 2014; 21:T273–83.
Article10. Dong W, Zhang H, Li J, Guan H, He L, Wang Z, et al. Estrogen induces metastatic potential of papillary thyroid cancer cells through estrogen receptor α and β. Int J Endocrinol. 2013; 2013:941568.11. Kumar A, Klinge CM, Goldstein RE. Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. Int J Oncol. 2010; 36:1067–80.12. Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008; 22:2085–98.13. Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014; 90:13–29.
Article14. Sun Y, Wang Y, Fan C, Gao P, Wang X, Wei G, et al. Estrogen promotes stemness and invasiveness of ER-positive breast cancer cells through Gli1 activation. Mol Cancer. 2014; 13:137.
Article15. Dey P, Barros RP, Warner M, Strom A, Gustafsson JA. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J Mol Endocrinol. 2013; 51:T61–74.
Article16. Yasar P, Ayaz G, User SD, Gupur G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reprod Med Biol. 2016; 16:4–20.
Article17. Huang Y, Dong W, Li J, Zhang H, Shan Z, Teng W. Differential expression patterns and clinical significance of estrogen receptor-α and β in papillary thyroid carcinoma. BMC Cancer. 2014; 14:383.
Article18. Yi JW, Kim SJ, Kim JK, Seong CY, Yu HW, Chai YJ, et al. Upregulation of the ESR1 gene and ESR ratio (ESR1/ESR2) is associated with a worse prognosis in papillary thyroid carcinoma: the impact of the estrogen receptor α/β expression on clinical outcomes in papillary thyroid carcinoma patients. Ann Surg Oncol. 2017; 24:3754–62.
Article19. Rajoria S, Suriano R, Shanmugam A, Wilson YL, Schantz SP, Geliebter J, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010; 20:33–41.
Article20. Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, et al. Association between BRAFV600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013; 309:1493–501.
Article21. Liu C, Chen T, Liu Z. Associations between BRAF(V600E) and prognostic factors and poor outcomes in papillary thyroid carcinoma: a meta-analysis. World J Surg Oncol. 2016; 14:241.
Article22. Lee SE, Hwang TS, Choi YL, Kim WY, Han HS, Lim SD, et al. Molecular profiling of papillary thyroid carcinoma in Korea with a high prevalence of BRAFV600E mutation. Thyroid. 2017; 27:802–10.
Article23. Atanaskova N, Keshamouni VG, Krueger JS, Schwartz JA, Miller F, Reddy KB. MAP kinase/estrogen receptor crosstalk enhances estrogen-mediated signaling and tumor growth but does not confer tamoxifen resistance. Oncogene. 2002; 21:4000–8.
Article24. Yu L, Moore AB, Castro L, Gao X, Huynh HL, Klippel M, et al. Estrogen regulates MAPK-related genes through genomic and nongenomic interactions between IGF-I receptor tyrosine kinase and estrogen receptor-alpha signaling pathways in human uterine leiomyoma cells. J Signal Transduct. 2012; 2012:204236.
Article25. Thomas RS, Sarwar N, Phoenix F, Coombes RC, Ali S. Phosphorylation at serines 104 and 106 by Erk1/2 MAPK is important for estrogen receptor-alpha activity. J Mol Endocrinol. 2008; 40:173–84.26. Marzagalli M, Montagnani Marelli M, Casati L, Fontana F, Moretti RM, Limonta P. Estrogen receptor β in melanoma: from molecular insights to potential clinical utility. Front Endocrinol (Lausanne). 2016; 7:140.
Article27. Topi G, Ghatak S, Satapathy SR, Ehrnstrom R, Lydrup ML, Sjolander A. Combined estrogen alpha and beta receptor expression has a prognostic significance for colorectal cancer patients. Front Med (Lausanne). 2022; 9:739620.
Article28. Kim BA, Jee HG, Yi JW, Kim SJ, Chai YJ, Choi JY, et al. Expression profiling of a human thyroid cell line stably expressing the BRAFV600E mutation. Cancer Genomics Proteomics. 2017; 14:53–67.
Article29. Kim M, Kim SJ, Xu Z, Ha SY, Byeon JH, Kang EJ, et al. BRAFV600E transduction of an SV40-immortalized normal human thyroid cell line induces dedifferentiated thyroid carcinogenesis in a mouse xenograft model. Thyroid. 2020; 30:487–500.
Article30. Marzagalli M, Casati L, Moretti RM, Montagnani Marelli M, Limonta P. Estrogen receptor β agonists differentially affect the growth of human melanoma cell lines. PLoS One. 2015; 10:e0134396.
Article31. Song P, Li Y, Dong Y, Liang Y, Qu H, Qi D, et al. Estrogen receptor β inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J Exp Clin Cancer Res. 2019; 38:354.
Article32. Zhou HB, Carlson KE, Stossi F, Katzenellenbogen BS, Katzenellenbogen JA. Analogs of methyl-piperidinopyrazole (MPP): antiestrogens with estrogen receptor alpha selective activity. Bioorg Med Chem Lett. 2009; 19:108–10.33. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.34. Mori S, Chang JT, Andrechek ER, Matsumura N, Baba T, Yao G, et al. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009; 28:2796–805.
Article35. Kim YN, Koo KH, Sung JY, Yun UJ, Kim H. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol. 2012; 2012:306879.
Article36. Santin AP, Furlanetto TW. Role of estrogen in thyroid function and growth regulation. J Thyroid Res. 2011; 2011:875125.
Article37. Crispo F, Notarangelo T, Pietrafesa M, Lettini G, Storto G, Sgambato A, et al. BRAF inhibitors in thyroid cancer: clinical impact, mechanisms of resistance and future perspectives. Cancers (Basel). 2019; 11:1388.
Article38. Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh QY, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246:466–71.
Article39. Weber CJ, Marvin M, Krekun S, Koschitzky T, Karp F, Benson M, et al. Effects of tamoxifen and somatostatin analogue on growth of human medullary, follicular, and papillary thyroid carcinoma cell lines: tissue culture and nude mouse xenograft studies. Surgery. 1990; 108:1065–71.40. Hoelting T, Duh QY, Clark OH, Herfarth C. Tamoxifen antagonizes proliferation and invasion of estrogen receptornegative metastatic follicular thyroid cancer cells via protein kinase C. Cancer Lett. 1996; 100:89–93.
Article41. de Araujo LF, Soares JM Jr, Simoes RS, Calio PL, OliveiraFilho RM, Simoes Mde J, et al. Effect of conjugated equine estrogens and tamoxifen administration on thyroid gland histomorphology of the rat. Clinics (Sao Paulo). 2006; 61:321–6.
Article42. Zidan J, Rubenstein W. Effect of adjuvant tamoxifen therapy on thyroid function in postmenopausal women with breast cancer. Oncology. 1999; 56:43–5.
Article43. Jiang D, Srinivasan A, Lozano G, Robbins PD. SV40 T antigen abrogates p53-mediated transcriptional activity. Oncogene. 1993; 8:2805–12.44. DeCaprio JA, Ludlow JW, Figge J, Shew JY, Huang CM, Lee WH, et al. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988; 54:275–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Implication of BRAF Mutation in Thyroid Cancer
- Expressions of miRNAs in Papillary Thyroid Carcinoma and Their Associations with the BRAFV600EMutation and Clinicopathological Features.
- Association of BRAF(V600E) Mutation with Poor Clinical Prognostic Factors and Ultrasonographic Findings in Cases of Papillary Thyroid Carcinoma
- Clinicopathological Implications of the BRAF(V600E) Mutation in PTC with Concurrent Hashimoto Thyroiditis
- Immunocytochemical analysis for estrogen receptors in the patients with thyroid disease