Korean J Gastroenterol.
2022 Apr;79(4):161-169. 10.4166/kjg.2022.003.
Tauroursodeoxycholic Acid Inhibits Nuclear Factor Kappa B Signaling in Gastric Epithelial Cells and Ameliorates Gastric Mucosal Damage in Mice
- Affiliations
-
- 1Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Pathology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Pathology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2529366
- DOI: http://doi.org/10.4166/kjg.2022.003
Abstract
- Background/Aims
Previous studies have reported the protective effects of tauroursodeoxycholic acid (TUDCA) on gastric epithelial cells in some animal models, but the precise mechanisms are unclear. This study examined the effects of TUDCA on NF-κB signaling in gastric epithelial cells. Moreover, the protective effects of TUDCA in experimental gastritis models induced by ethanol and NSAID were evaluated and compared with ursodeoxycholic acid (UDCA).
Methods
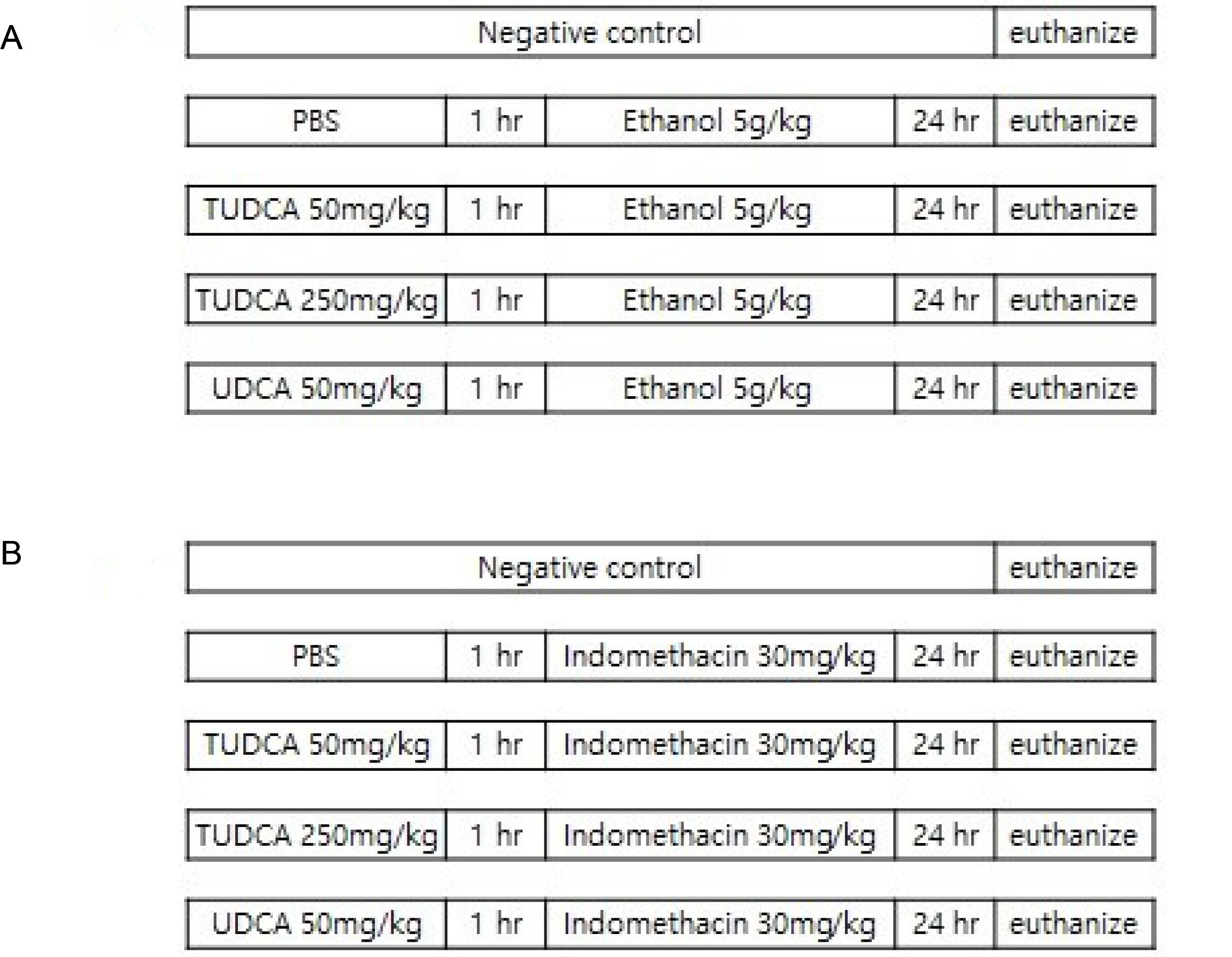
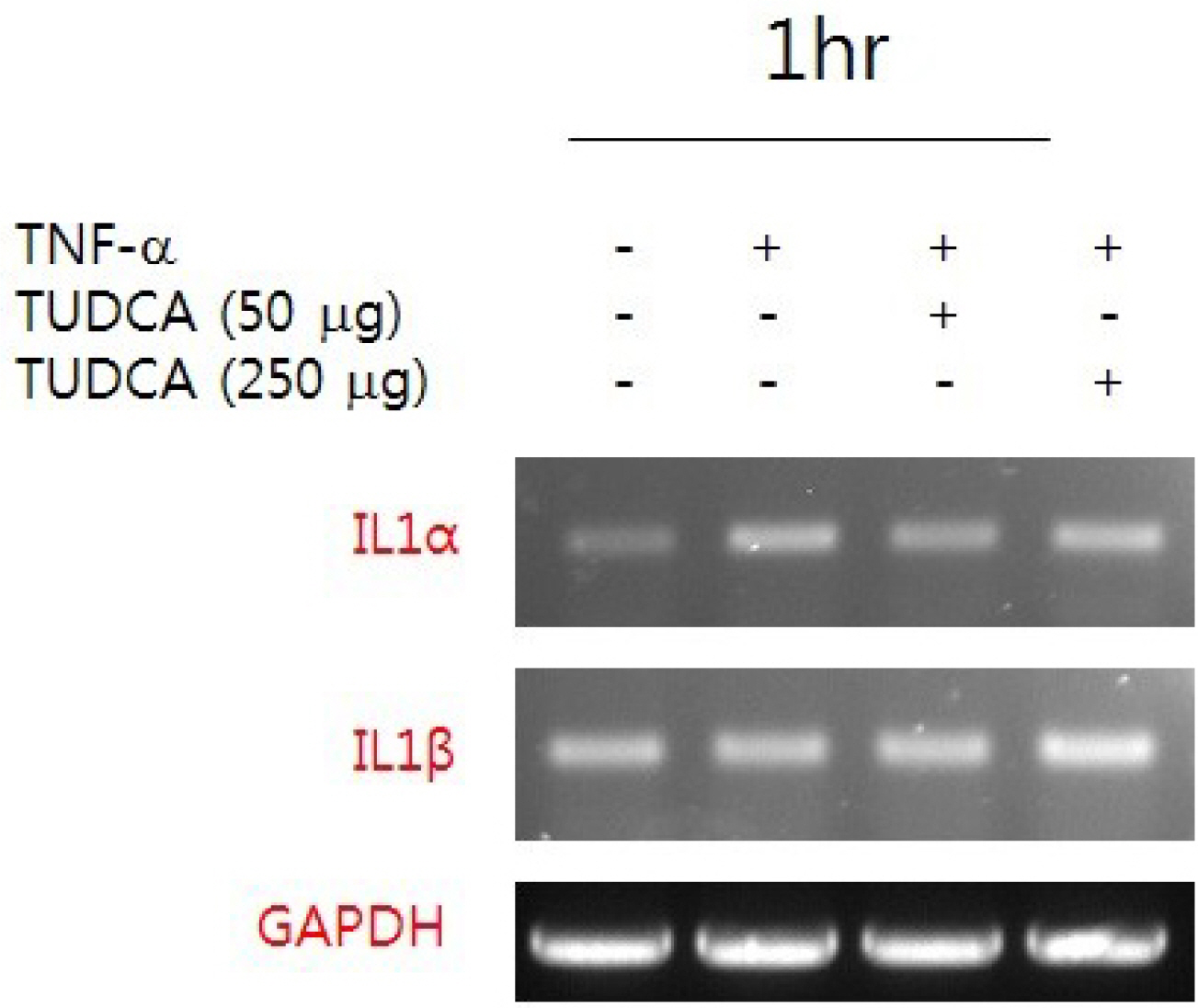
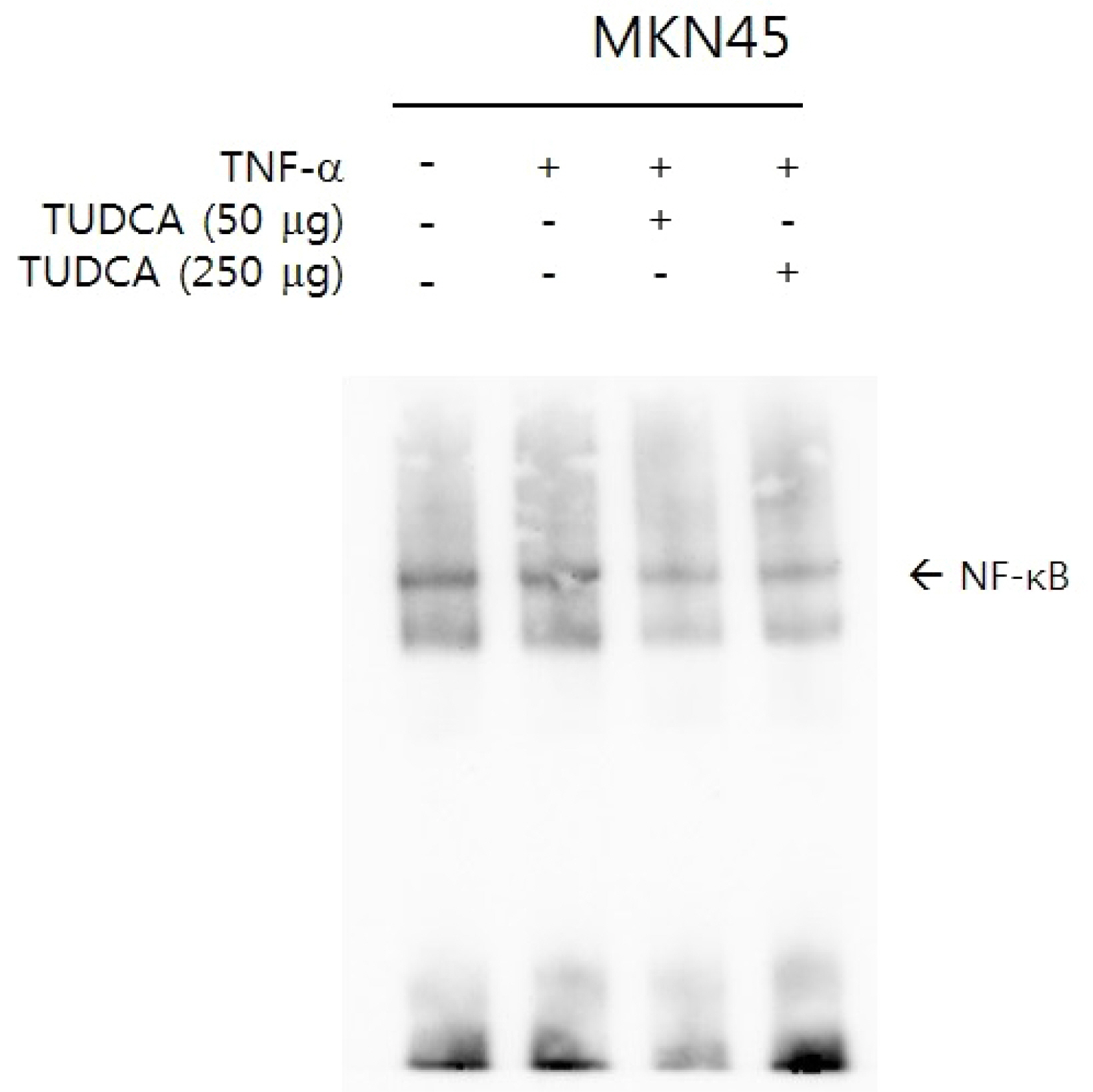
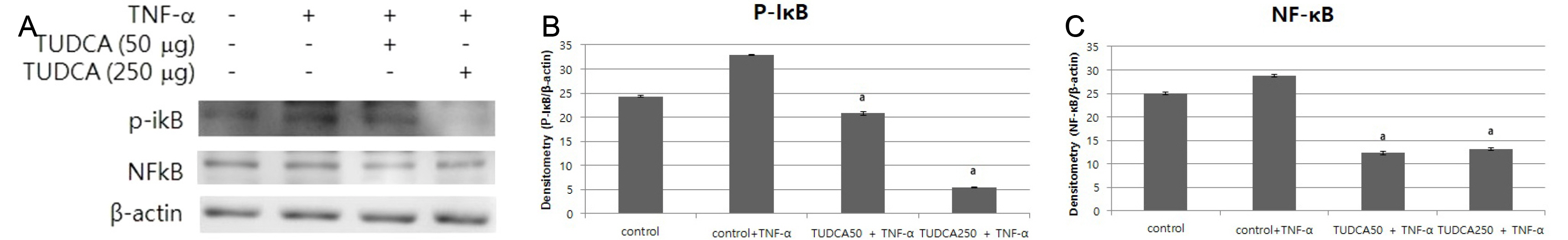
After a pretreatment with TUDCA or UDCA, human gastric epithelial MKN-45 cells were stimulated with tumor necrosis factor (TNF)-α to activate NF-κB signaling. A real-time PCR (RT-PCR) for human interleukin (IL)-1 mRNA was performed. An electrophoretic mobility shift assay (EMSA) and immunoblot analyses were carried out. In murine models, after a pretreatment with TUDCA or UDCA, ethanol and indomethacin were administered via oral gavage. Macroscopic and microscopic assessments were performed to evaluate the preventive effects of TUDCA and UDCA on murine gastritis.
Results
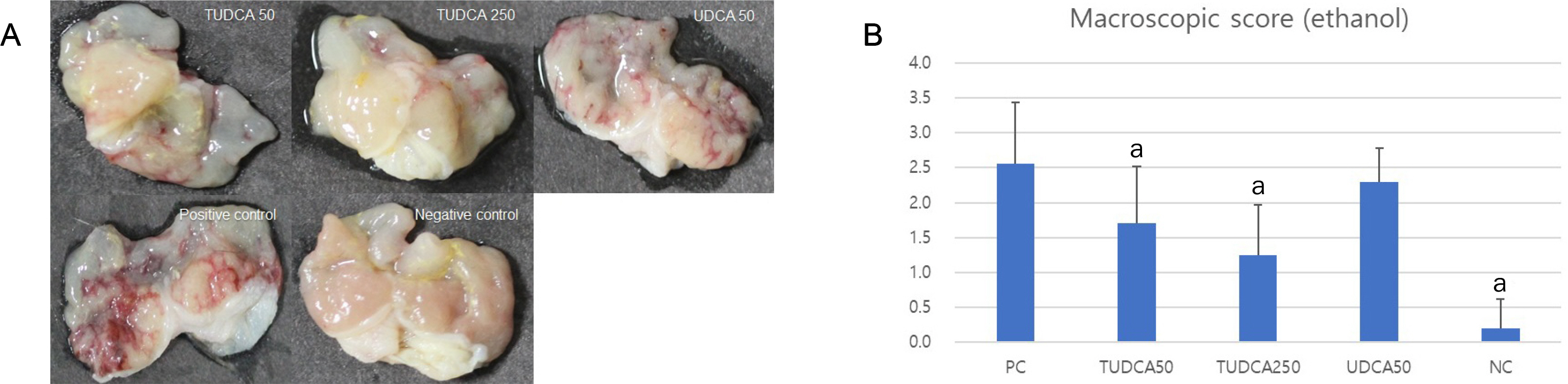
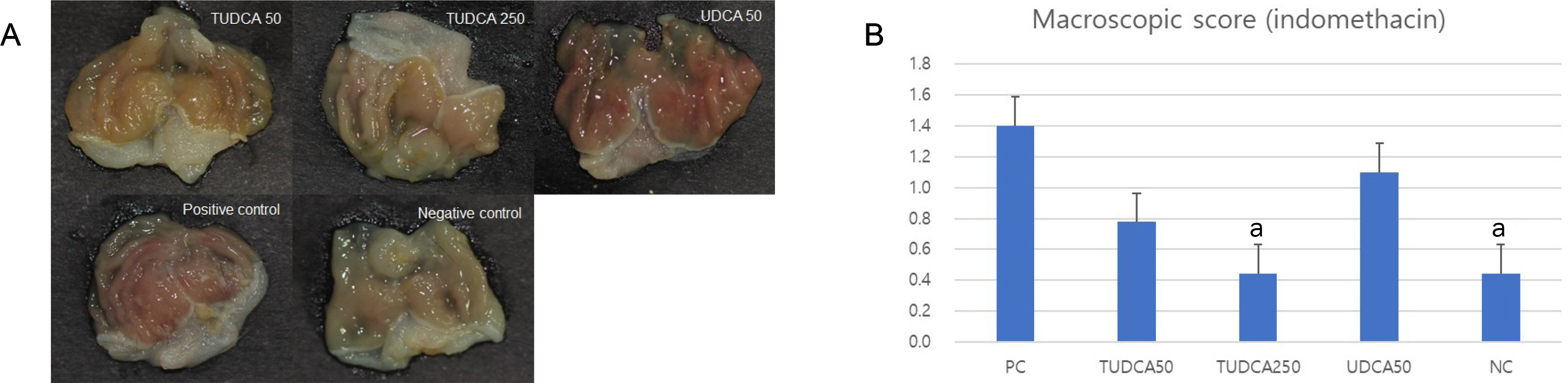
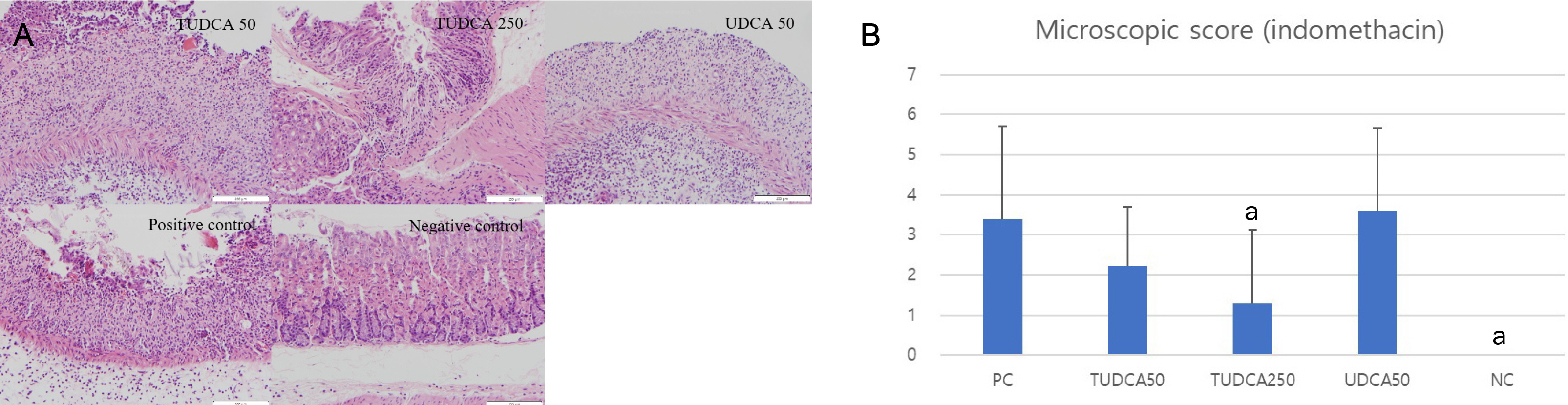
A pretreatment with TUDCA downregulated the IL-1α mRNA levels in MKN-45 cells stimulated with TNF-α, as assessed by RT-PCR. As determined using EMSA, a pretreatment with TUDCA reduced the TNF-α-induced NF-κB DNA binding activity. A pretreatment with TUDCA inhibited IκBα phosphorylation induced by TNF-α, as assessed by immunoblot analysis. TUDCA attenuated the ethanol-induced and NSAID-induced gastritis in murine models, as determined macroscopically and microscopically.
Conclusions
TUDCA inhibited NF-κB signaling in gastric epithelial cells and ameliorated ethanol- and NSAID-induced gastritis in murine models. These results support the potential of TUDCA for the prevention of gastritis in humans.
Figure
Reference
-
1. Choi SM, Shin JH, Kang KK, Ahn BO, Yoo M. 2007; Gastroprotective effects of DA-6034, a new flavonoid derivative, in various gastric mucosal damage models. Dig Dis Sci. 52:3075–3080. DOI: 10.1007/s10620-006-9657-4. PMID: 17406830.
Article2. Jönsson KA, Gotthard R, Bodemar G, Brodin U. 1989; The clinical relevance of endoscopic and histologic inflammation of gastroduodenal mucosa in dyspepsia of unknown origin. Scand J Gastroenterol. 24:385–395. DOI: 10.3109/00365528909093064. PMID: 2675301.
Article3. Quach DT, Hiyama T. 2019; Assessment of endoscopic gastric atrophy according to the Kimura-Takemoto classification and its potential application in daily practice. Clin Endosc. 52:321–327. DOI: 10.5946/ce.2019.072. PMID: 31327182. PMCID: PMC6680010.
Article4. Nahid-Samiei M, Rahimian G, Shafigh M, et al. 2020; Enhanced frequency of CD19+IL-10+B cells in human gastric mucosa infected by Helicobacter pylori. Am J Med Sci. 359:347–353. DOI: 10.1016/j.amjms.2020.03.019. PMID: 32354596.
Article5. Franke A, Teyssen S, Singer MV. 2005; Alcohol-related diseases of the esophagus and stomach. Dig Dis. 23:204–213. DOI: 10.1159/000090167. PMID: 16508284.
Article6. Guo CG, Leung WK. 2020; Potential strategies in the prevention of nonsteroidal anti-inflammatory drugs-associated adverse effects in the lower gastrointestinal tract. Gut Liver. 14:179–189. DOI: 10.5009/gnl19201. PMID: 31547642. PMCID: PMC7096237.
Article7. Laukens D, Devisscher L, Van den Bossche L, et al. 2014; Tauroursodeoxycholic acid inhibits experimental colitis by preventing early intestinal epithelial cell death. Lab Invest. 94:1419–1430. DOI: 10.1038/labinvest.2014.117. PMID: 25310532.
Article8. Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. 2009; Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 50:1721–1734. DOI: 10.1194/jlr.R900011-JLR200. PMID: 19417220. PMCID: PMC2724780.
Article9. Kim YH, Kim JH, Kim BG, Lee KL, Kim JW, Koh SJ. 2019; Tauroursodeoxycholic acid attenuates colitis-associated colon cancer by inhibiting nuclear factor kappaB signaling. J Gastroenterol Hepatol. 34:544–551. DOI: 10.1111/jgh.14526. PMID: 30378164.
Article10. Ota S, Tsukahara H, Terano A, et al. 1991; Protective effect of tauroursodeoxycholate against chenodeoxycholate-induced damage to cultured rabbit gastric cells. Dig Dis Sci. 36:409–416. DOI: 10.1007/BF01298867. PMID: 2007357.
Article11. Piepoli AL, Caroppo R, Armentano R, Caruso ML, Guerra V, Maselli MA. 2002; Tauroursodeoxycholic acid reduces damaging effects of taurodeoxycholic acid on fundus gastric mucosa. Arch Physiol Biochem. 110:197–202. DOI: 10.1076/apab.110.3.197.8295. PMID: 12221520.
Article12. Kim JM, Kim SH, Ko SH, et al. 2013; The guggulsterone derivative GG-52 inhibits NF-κB signaling in gastric epithelial cells and ameliorates ethanol-induced gastric mucosal lesions in mice. Am J Physiol Gastrointest Liver Physiol. 304:G193–G202. DOI: 10.1152/ajpgi.00103.2012. PMID: 23125156.
Article13. Koh SJ, Kim JW, Kim BG, Lee KL, Chun J, Kim JS. 2015; Fexofenadine regulates nuclear factor-κB signaling and endoplasmic reticulum stress in intestinal epithelial cells and ameliorates acute and chronic colitis in mice. J Pharmacol Exp Ther. 352:455–461. DOI: 10.1124/jpet.114.217844. PMID: 25538104.
Article14. Koh SJ, Kim JM, Kim IK, Ko SH, Kim JS. 2014; Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J Gastroenterol Hepatol. 29:502–510. DOI: 10.1111/jgh.12435. PMID: 24716225.
Article15. Festing MF. 2006; Design and statistical methods in studies using animal models of development. ILAR J. 47:5–14. DOI: 10.1093/ilar.47.1.5. PMID: 16391426.
Article16. Festing MF, Altman DG. 2002; Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 43:244–258. DOI: 10.1093/ilar.43.4.244. PMID: 12391400.
Article17. Park JM, Han YM, Kangwan N, et al. 2014; S-allyl cysteine alleviates nonsteroidal anti-inflammatory drug-induced gastric mucosal damages by increasing cyclooxygenase-2 inhibition, heme oxygenase-1 induction, and histone deacetylation inhibition. J Gastroenterol Hepatol. 29(Suppl 4):80–92. DOI: 10.1111/jgh.12730. PMID: 25521739.
Article18. Jobin C, Sartor RB. 2000; The I kappa B/NF-kappa B system: a key determinant of mucosalinflammation and protection. Am J Physiol Cell Physiol. 278:C451–C462. DOI: 10.1152/ajpcell.2000.278.3.C451. PMID: 10712233.19. Berger E, Haller D. 2011; Structure-function analysis of the tertiary bile acid TUDCA for the resolution of endoplasmic reticulum stress in intestinal epithelial cells. Biochem Biophys Res Commun. 409:610–615. DOI: 10.1016/j.bbrc.2011.05.043. PMID: 21605547.
Article20. Ma TY, Iwamoto GK, Hoa NT, et al. 2004; TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 286:G367–G376. DOI: 10.1152/ajpgi.00173.2003. PMID: 14766535.21. Karin M, Greten FR. 2005; NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 5:749–759. DOI: 10.1038/nri1703. PMID: 16175180.
Article22. Aggarwal BB. 2004; Nuclear factor-kappaB: the enemy within. Cancer Cell. 6:203–208. DOI: 10.1016/j.ccr.2004.09.003. PMID: 15380510.23. Yang YX, Metz DC. 2010; Safety of proton pump inhibitor exposure. Gastroenterology. 139:1115–1127. DOI: 10.1053/j.gastro.2010.08.023. PMID: 20727892.
Article24. Fackler WK, Ours TM, Vaezi MF, Richter JE. 2002; Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology. 122:625–632. DOI: 10.1053/gast.2002.31876. PMID: 11874994.
Article25. Aihara T, Nakamura E, Amagase K, et al. 2003; Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: past, present, and future. Pharmacol Ther. 98:109–127. DOI: 10.1016/S0163-7258(03)00015-9.
Article26. Sarkar M, Hennessy S, Yang YX. 2008; Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 149:391–398. DOI: 10.7326/0003-4819-149-6-200809160-00005. PMID: 18794558.
Article27. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. 2012; Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 107:1001–1010. DOI: 10.1038/ajg.2012.179. PMID: 22710578.
Article28. Choudhry U, Boyce HW Jr, Coppola D. 1998; Proton pump inhibitor-associated gastric polyps: a retrospective analysis of their frequency, and endoscopic, histologic, and ultrastructural characteristics. Am J Clin Pathol. 110:615–621. DOI: 10.1093/ajcp/110.5.615. PMID: 9802346.
Article29. Kim GH. 2021; Proton pump inhibitor-related gastric mucosal changes. Gut Liver. 15:646–652. DOI: 10.5009/gnl20036. PMID: 32327613. PMCID: PMC8444106.
Article30. Kwon JH, Koh SJ, Kim W, et al. 2014; Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol. 29:775–781. DOI: 10.1111/jgh.12426. PMID: 24219827.
Article31. Min YW, Lim KS, Min BH, et al. 2014; Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther. 40:695–704. DOI: 10.1111/apt.12875. PMID: 25078671.
Article32. Singer MV, Leffmann C, Eysselein VE, Calden H, Goebell H. 1987; Action of ethanol and some alcoholic beverages on gastric acid secretion and release of gastrin in humans. Gastroenterology. 93:1247–1254. DOI: 10.1016/0016-5085(87)90252-6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trefoil Factor 1 Suppresses Epithelial-mesenchymal Transition through Inhibition of TGF-beta Signaling in Gastric Cancer Cells
- Helicobacter pylori-associated Chronic Atrophic Gastritis and Progression of Gastric Carcinogenesis
- Astaxanthin Inhibits Helicobacter pylori-induced Inflammatory and Oncogenic Responses in Gastric Mucosal Tissues of Mice
- Effect of Meloxicam, Selective COX-2 Inhibitor, on Gastric Mucosal Damage and Serum TNF-alpha
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells