Nutr Res Pract.
2021 Dec;15(6):798-806. 10.4162/nrp.2021.15.6.798.
Dietary glucosinolates inhibit splenic inflammation in high fat/cholesterol diet-fed C57BL/6 mice
- Affiliations
-
- 1Department of Food and Nutrition, Chonnam National University, Gwangju 61186, Korea
- 2Department of Education, Graduate School of Education, Chonnam National University, Gwangju 61186, Korea
- KMID: 2522658
- DOI: http://doi.org/10.4162/nrp.2021.15.6.798
Abstract
- BACKGROUND/OBJECTIVES
Obesity is associated with chronic inflammation. The spleen is the largest organ of the lymphatic system and has an important role in immunity. Obesity-induced inflammatory responses are triggered by Toll-like receptor (TLR)-myeloid differentiation primary response 88 (MyD88) pathway signaling. Phenethyl isothiocyanate (PEITC) and 3,3′-diindolylmethane (DIM), major dietary glucosinolates present in cruciferous vegetables, have been reported to produce anti-inflammatory effects on various diseases. However, the effects of PEITC and DIM on the obesity-induced inflammatory response in the spleen are unclear. The purpose of this study was to examine the antiinflammatory effects of PEITC and DIM on the spleen and their mechanism in high fat/ cholesterol diet (HFCD)-fed C57BL/6 mice.
MATERIALS/METHODS
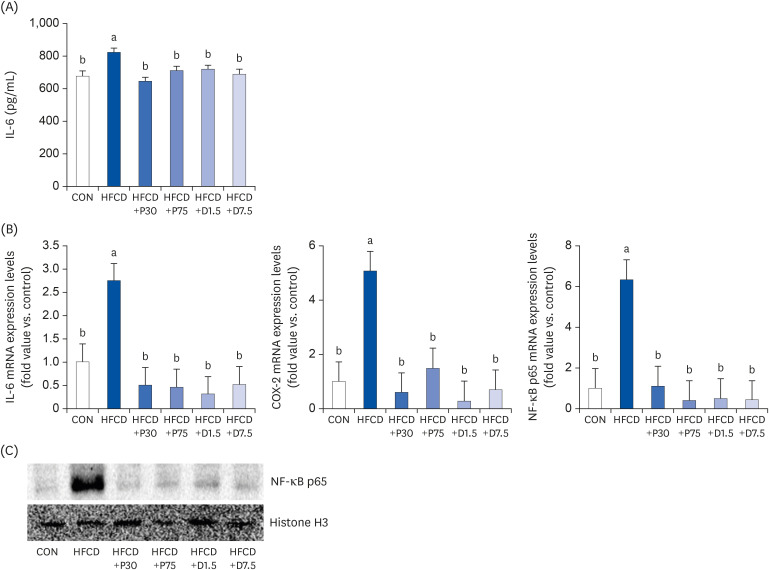
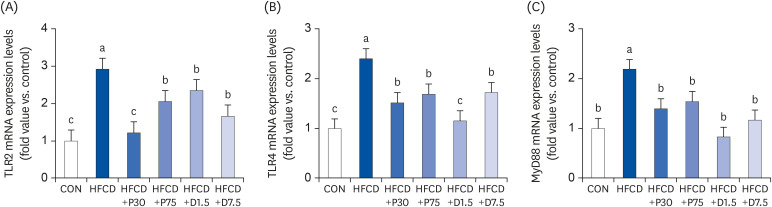
We established an animal model of HFCD-induced obesity using C57BL/6 mice. The mice were divided into six groups: normal diet with AIN-93G diet (CON), high fat diet (60% calories from fat) with 1% cholesterol (HFCD), HFCD with PEITC 30 mg/kg/ day or 75 mg/kg/day (HFCD+P30, HFCD+P75), and HFCD with DIM 1.5 mg/kg/day or 7.5 mg/kg/ day (HFCD+D1.5, HFCD+D7.5). Enzyme-linked immunosorbent assay was used to evaluate proinflammatory cytokine secretion. Western blot and quantitative polymerase chain reaction were used to analyze protein and mRNA levels of nuclear factor kappa B (NF-κB) p65, interleukin 6 (IL-6), cyclooxygenase 2 (COX-2), TLR2, TLR4, and MyD88 in spleen tissue.
RESULTS
Serum IL-6 levels were significantly higher in the HFCD group than in groups fed a HFCD with PEITC or DIM. Levels of NF-κB p65 protein and TLR2/4, MyD88, NF-κB p65, IL-6, and COX-2 mRNA were significantly higher in the HFCD group than in the CON group and were reduced by the PEITC and DIM supplements.
CONCLUSIONS
PEITC- and DIM-supplemented diets improved splenic inflammation by modulating the TLR2/4-MyD88 pathway in HFCD-fed mice. We suggest that dietary glucosinolates may at least partially improve obesity-induced inflammation of the spleen.
Keyword
Figure
Reference
-
1. Kortt MA, Clarke PM. Estimating utility values for health states of overweight and obese individuals using the SF-36. Qual Life Res. 2005; 14:2177–2185. PMID: 16328898.
Article2. Magnuson AM, Regan DP, Fouts JK, Booth AD, Dow SW, Foster MT. Diet-induced obesity causes visceral, but not subcutaneous, lymph node hyperplasia via increases in specific immune cell populations. Cell Prolif. 2017; 50:50.3. Inoue M, Gotoh K, Seike M, Masaki T, Honda K, Kakuma T, Yoshimatsu H. Role of the spleen in the development of steatohepatitis in high-fat-diet-induced obese rats. Exp Biol Med (Maywood). 2012; 237:461–470. PMID: 22490513.
Article4. Zhang C, Li C, Jia X, Wang K, Tu Y, Wang R, Liu K, Lu T, He C. In vitro and in vivo anti-inflammatory effects of polyphyllin VII through downregulating MAPK and NF-κB pathways. Molecules. 2019; 24:24.5. Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000; 148:209–214. PMID: 10657556.
Article6. Zhu YJ, Wang C, Song G, Zang SS, Liu YX, Li L. Toll-like receptor-2 and -4 are associated with hyperlipidemia. Mol Med Rep. 2015; 12:8241–8246. PMID: 26497845.
Article7. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014; 5:461. PMID: 25309543.
Article8. Kleinridders A, Schenten D, Könner AC, Belgardt BF, Mauer J, Okamura T, Wunderlich FT, Medzhitov R, Brüning JC. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009; 10:249–259. PMID: 19808018.
Article9. Song J, Kim YS, Kim L, Park HJ, Lee D, Kim H. Anti-obesity effects of the flower of Prunus persica in high-fat diet-induced obese mice. Nutrients. 2019; 11:11.10. Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002; 76:1191–1201. PMID: 12450882.
Article11. Ryu Y, Jin L, Kee HJ, Piao ZH, Cho JY, Kim GR, Choi SY, Lin MQ, Jeong MH. Gallic acid prevents isoproterenol-induced cardiac hypertrophy and fibrosis through regulation of JNK2 signaling and Smad3 binding activity. Sci Rep. 2016; 6:34790. PMID: 27703224.
Article12. Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation is accompanied by activation of Wnt/beta-catenin signaling. Am J Physiol Cell Physiol. 2010; 298:C1510–6. PMID: 20357182.13. Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008; 373:545–549. PMID: 18586010.
Article14. Ranjan A, Ramachandran S, Gupta N, Kaushik I, Wright S, Srivastava S, Das H, Srivastava S, Prasad S, Srivastava SK. Role of phytochemicals in cancer prevention. Int J Mol Sci. 2019; 20:4981.
Article15. Gupta P, Wright SE, Kim SH, Srivastava SK. Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms. Biochim Biophys Acta. 2014; 1846:405–424. PMID: 25152445.
Article16. Tian X, Liu K, Zu X, Ma F, Li Z, Lee M, Chen H, Li Y, Zhao Y, Liu F, Oi N, Bode AM, Dong Z, Kim DJ. 3,3′-Diindolylmethane inhibits patient-derived xenograft colon tumor growth by targeting COX1/2 and ERK1/2. Cancer Lett. 2019; 448:20–30. PMID: 30716361.
Article17. Yao Z, Hu W, Yin S, Huang Z, Zhu Q, Chen J, Zang Y, Dong L, Zhang J. 3,3′-Diindolymethane ameliorates adriamycin-induced cardiac fibrosis via activation of a BRCA1-dependent anti-oxidant pathway. Pharmacol Res. 2013; 70:139–146. PMID: 23376355.
Article18. Kim SM. Cellular and molecular mechanisms of 3,3′-diindolylmethane in gastrointestinal cancer. Int J Mol Sci. 2016; 17:1155.
Article19. Gwon MH, Im YS, Seo AR, Kim KY, Moon HR, Yun JM. Phenethyl isothiocyanate protects against high fat/cholesterol diet-induced obesity and atherosclerosis in C57BL/6 Mice. Nutrients. 2020; 12:3657.
Article20. Antoniotti GS, Coughlan M, Salamonsen LA, Evans J. Obesity associated advanced glycation end products within the human uterine cavity adversely impact endometrial function and embryo implantation competence. Hum Reprod. 2018; 33:654–665. PMID: 29471449.
Article21. Venosa A, Malaviya R, Gow AJ, Hall L, Laskin JD, Laskin DL. Protective role of spleen-derived macrophages in lung inflammation, injury, and fibrosis induced by nitrogen mustard. Am J Physiol Lung Cell Mol Physiol. 2015; 309:L1487–98. PMID: 26475734.
Article22. Rogero MM, Calder PC. Obesity, inflammation, Toll-like receptor 4 and fatty acids. Nutrients. 2018; 10:432.
Article23. Tarantino G, Savastano S, Capone D, Colao A. Spleen: a new role for an old player? World J Gastroenterol. 2011; 17:3776–3784. PMID: 21987619.
Article24. Fang Y, Wang S, Zhu T, Zhang Y, Lian X. Atherogenic high cholesterol/high fat diet induces TLRs-associated pulmonary inflammation in C57BL/6J mice. Inflamm Res. 2017; 66:39–47. PMID: 27653962.
Article25. Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010; 23:270–299. PMID: 20977819.
Article26. Gotoh K, Inoue M, Masaki T, Chiba S, Shimasaki T, Ando H, Fujiwara K, Katsuragi I, Kakuma T, Seike M, Sakata T, Yoshimatsu H. A novel anti-inflammatory role for spleen-derived interleukin-10 in obesity-induced inflammation in white adipose tissue and liver. Diabetes. 2012; 61:1994–2003. PMID: 22648387.
Article27. Altunkaynak BZ, Ozbek E, Altunkaynak ME. A stereological and histological analysis of spleen on obese female rats, fed with high fat diet. Saudi Med J. 2007; 28:353–357. PMID: 17334458.28. Trufakin VA, Shurlygina AV, Dushkin MI, Khrapova MV, Michurina SV, Mel’nikova EV, Panteleeva NG, Tenditnik MV. Effect of melatonin on cellular composition of the spleen and parameters of lipid metabolism in rats with alimentary obesity. Bull Exp Biol Med. 2014; 158:42–45. PMID: 25403394.
Article29. Kuo JJ, Chang HH, Tsai TH, Lee TY. Positive effect of curcumin on inflammation and mitochondrial dysfunction in obese mice with liver steatosis. Int J Mol Med. 2012; 30:673–679. PMID: 22751848.
Article30. Carneiro NV, Silva HB, Silva RR, Carneiro TC, Costa RS, Pires AO, Marques CR, Velozo ES, Conceição AS, Silva TM, Silva TM, Alcântara-Neves NM, Figueiredo CA. Sambucus australis modulates inflammatory response via inhibition of nuclear factor kappa B (NF-kB) in vitro . An Acad Bras Cienc. 2019; 91:e20170831. PMID: 30916148.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Intermittent Fasting on Splenic Galectin-3 Protein Expression in High-fat Diet-fed Mice
- Anti-obesity effects of Rapha diet(R) preparation in mice fed a high-fat diet
- Anti-obesity and LDL-cholesterol lowering effects of silkworm hemolymph in C57BL/6N mice fed high fat diet
- Effects of pectin lyase-modified red ginseng extracts in high-fat diet-fed obese mice
- Effects of Calcium and Genistein on Body Fat and Lipid Metabolism in High Fat-induced Obese Mice