J Stroke.
2021 Sep;23(3):420-436. 10.5853/jos.2021.00626.
Neuroprotective Effects of GV1001 in Animal Stroke Model and Neural Cells Subject to Oxygen-Glucose Deprivation/Reperfusion Injury
- Affiliations
-
- 1Department of Neurology, Hanyang University Guri Hospital, Hangyang University College of Medicine, Guri, Korea
- 2Department of Neurology, Hanyang University Seoul Hospital, Hanyang University College of Medicine, Seoul, Korea
- 3Department of Translational Medicine, Hanyang University Graduate School of Biomedical Science & Engineering, Seoul, Korea
- KMID: 2520919
- DOI: http://doi.org/10.5853/jos.2021.00626
Abstract
- Background and Purpose
Previous studies have revealed the diverse neuroprotective effects of GV1001. In this study, we investigated the effects of GV1001 on focal cerebral ischemia-reperfusion injury (IRI) in rats and oxygen-glucose deprivation/reoxygenation (OGD/R)-induced injury in neural stem cells (NSCs) and cortical neurons.
Methods
Focal cerebral IRI was induced by transient middle cerebral artery occlusion (MCAO). Brain diffusion-weighted imaging (DWI) was performed 2 hours after occlusion, and a total of 37 rats were treated by reperfusion with GV1001 or saline 2 hours after occlusion. Fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging, immunohistochemistry, and neurobehavioral function analyses were performed. Additionally, OGD/R-injured NSCs and cortical neurons were treated with different GV1001 concentrations. Cell viability, proliferation, migration, and oxidative stress were determined by diverse molecular analyses.
Results
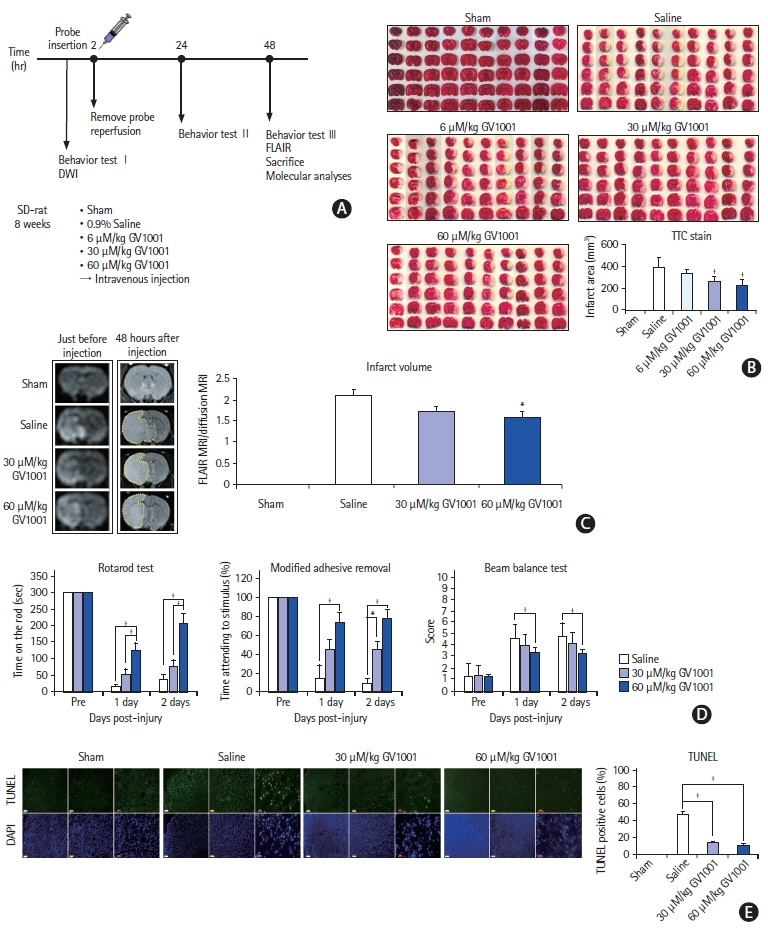
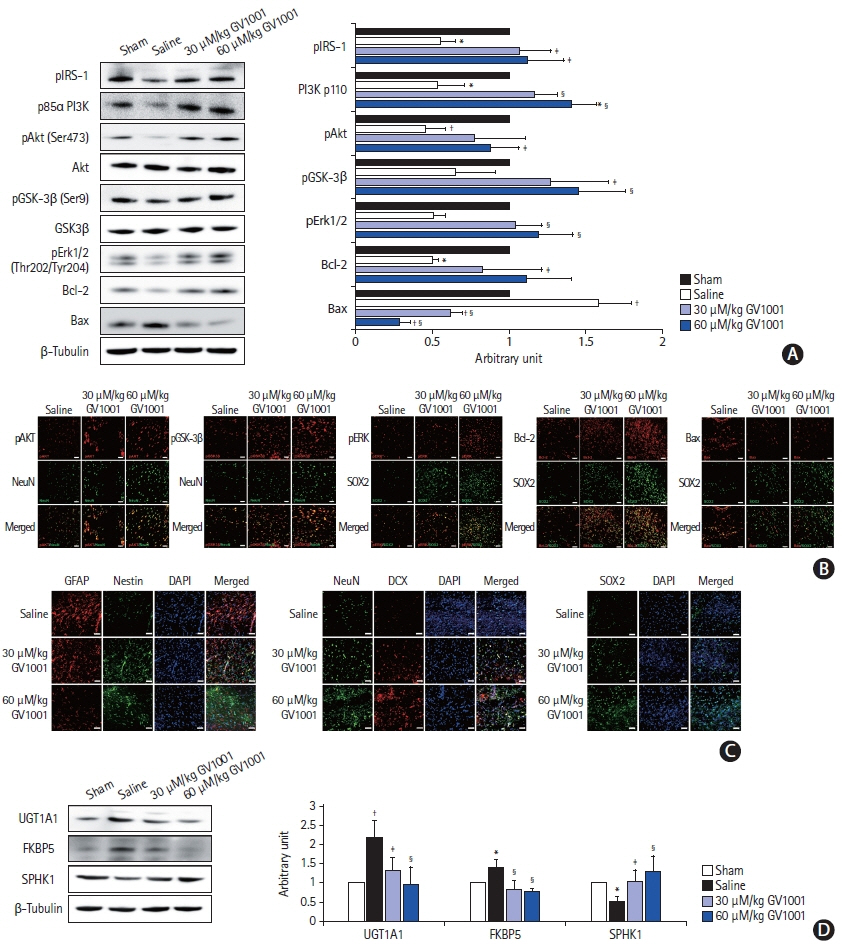
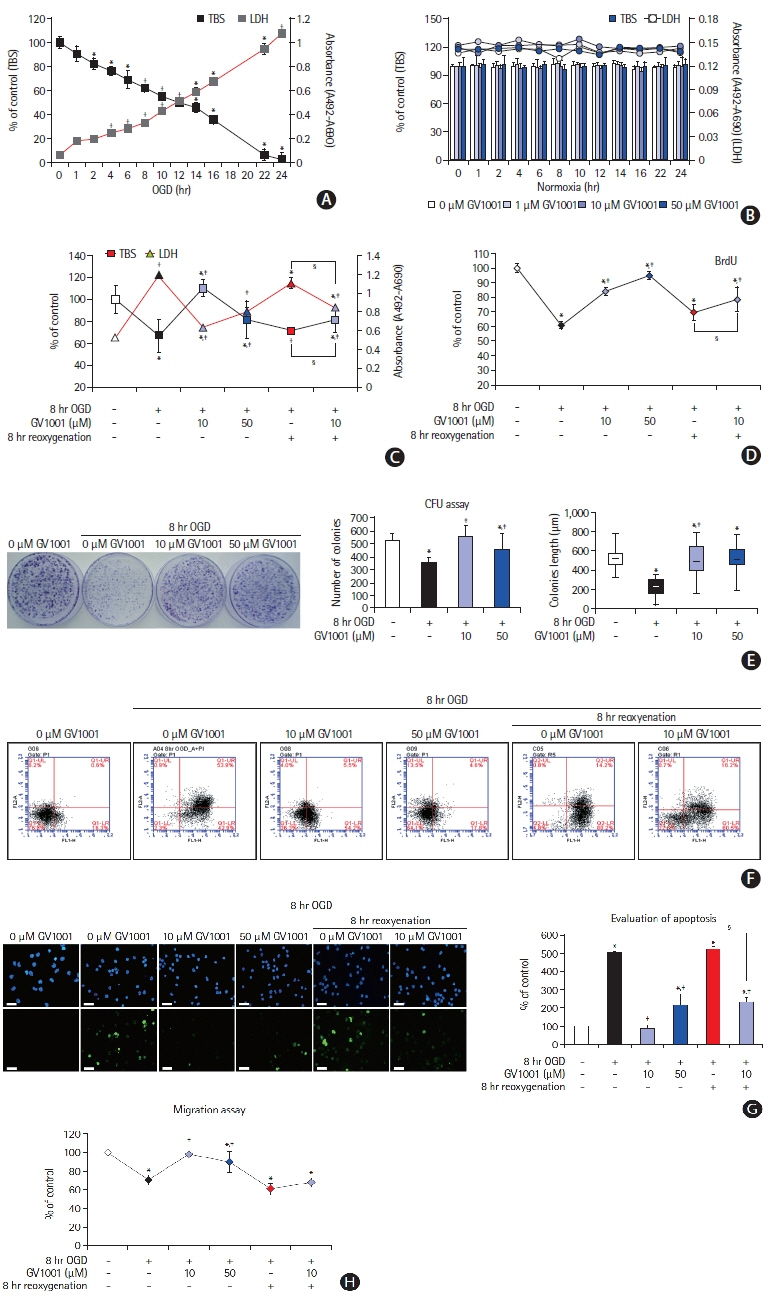
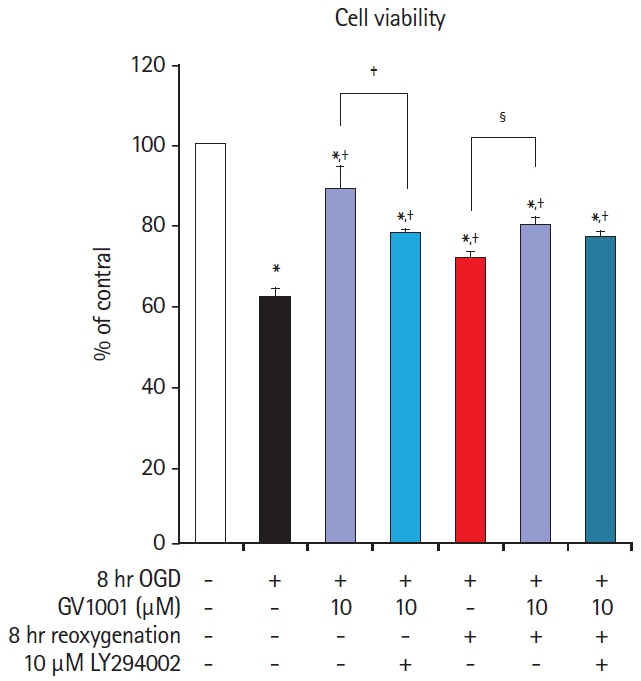
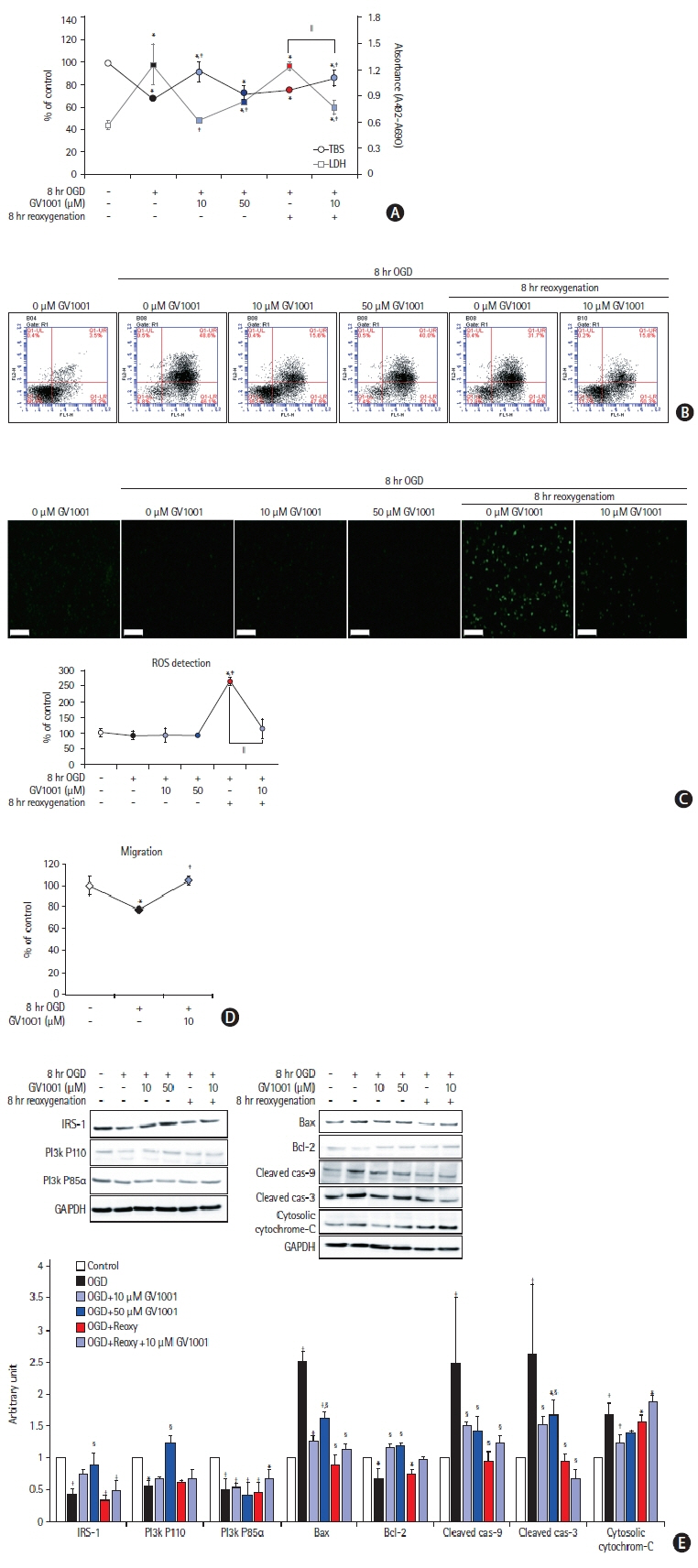
In the stroke model, GV1001 protected neural cells against IRI. The most effective dose of GV1001 was 60 μM/kg. The infarct volume on FLAIR 48 hours after MCAO compared to lesion volume on DWI showed a significantly smaller ratio in the GV1001-treated group. GV1001-treated rats exhibited better behavioral functions than the saline-treated rats. Treatment with GV1001 increased the viability, proliferation, and migration of the OGD/R-injured NSCs. Free radicals were significantly restored by treatment with GV1001. These neuroprotective effects of GV1001 have also been demonstrated in OGD/R-injured cortical neurons. Conclusions The results suggest that GV1001 has neuroprotective effects against IRI in NSCs, cortical neurons, and the rat brain. These effects are mediated through the induction of cellular proliferation, mitochondrial stabilization, and anti-apoptotic, anti-aging, and antioxidant effects.
Figure
Reference
-
References
1. Vidale S, Consoli A, Arnaboldi M, Consoli D. Postischemic Inflammation in Acute Stroke. J Clin Neurol. 2017; 13:1–9.
Article2. Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010; 41:172–179.
Article3. Park HH, Yu HJ, Kim S, Kim G, Choi NY, Lee EH, et al. Neural stem cells injured by oxidative stress can be rejuvenated by GV1001, a novel peptide, through scavenging free radicals and enhancing survival signals. Neurotoxicology. 2016; 55:131–141.
Article4. Martínez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat Rev Cancer. 2011; 11:161–176.
Article5. Park HH, Lee KY, Kim S, Lee JW, Choi NY, Lee EH, et al. Novel vaccine peptide GV1001 effectively blocks β-amyloid toxicity by mimicking the extra-telomeric functions of human telomerase reverse transcriptase. Neurobiol Aging. 2014; 35:1255–1274.6. Ko YJ, Kwon KY, Kum KY, Lee WC, Baek SH, Kang MK, et al. The anti-inflammatory effect of human telomerase-derived peptide on P. gingivalis lipopolysaccharide-induced inflammatory cytokine production and its mechanism in human dental pulp cells. Mediators Inflamm. 2015; 2015:385127.7. Koh SH, Park Y, Song CW, Kim JG, Kim K, Kim J, et al. The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci. 2004; 20:1461–1472.
Article8. Koh SH, Yoo AR, Chang DI, Hwang SJ, Kim SH. Inhibition of GSK-3 reduces infarct volume and improves neurobehavioral functions. Biochem Biophys Res Commun. 2008; 371:894–899.
Article9. Kim YS, Yoo A, Son JW, Kim HY, Lee YJ, Hwang S, et al. Early activation of phosphatidylinositol 3-kinase after ischemic stroke reduces infarct volume and improves long-term behavior. Mol Neurobiol. 2017; 54:5375–5384.
Article10. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001; 32:1005–1011.
Article11. Sughrue ME, Mocco J, Komotar RJ, Mehra A, D’Ambrosio AL, Grobelny BT, et al. An improved test of neurological dysfunction following transient focal cerebral ischemia in rats. J Neurosci Methods. 2006; 151:83–89.
Article12. Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998; 1:290–295.
Article13. Currle DS, Hu JS, Kolski-Andreaco A, Monuki ES. Culture of mouse neural stem cell precursors. J Vis Exp. 2007; 2:152.
Article14. Koo TY, Yan JJ, Yang J. Protective effect of peptide GV1001 against renal ischemia-reperfusion injury in mice. Transplant Proc. 2014; 46:1117–1122.
Article15. Kyte JA. Cancer vaccination with telomerase peptide GV1001. Expert Opin Investig Drugs. 2009; 18:687–694.
Article16. Lee YK, Nata’atmaja BS, Kim BH, Pak CS, Heo CY. Protective effect of telomerase-based 16-mer peptide vaccine (GV1001) on inferior epigastric island skin flap survivability in ischaemia-reperfusion injury rat model. J Plast Surg Hand Surg. 2017; 51:210–216.
Article17. Chang JE, Kim HJ, Jheon S, Lim C. Protective effects of GV1001 on myocardial ischemia-reperfusion injury. Mol Med Rep. 2017; 16:7315–7320.
Article18. Chang JE, Kim HJ, Yi E, Jheon S, Kim K. Reduction of ischaemiareperfusion injury in a rat lung transplantation model by low-concentration GV1001. Eur J Cardiothorac Surg. 2016; 50:972–979.
Article19. Son JW, Choi H, Yoo A, Park HH, Kim YS, Lee KY, et al. Activation of the phosphatidylinositol 3-kinase pathway plays important roles in reduction of cerebral infarction by cilnidipine. J Neurochem. 2015; 135:186–193.
Article20. Pap M, Cooper GM. Role of translation initiation factor 2B in control of cell survival by the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase 3beta signaling pathway. Mol Cell Biol. 2002; 22:578–586.21. Hemmings BA, Restuccia DF. The PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2015; 7:a026609.
Article22. Song WJ, Song EA, Jung MS, Choi SH, Baik HH, Jin BK, et al. Phosphorylation and inactivation of glycogen synthase kinase 3β (GSK3β) by dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). J Biol Chem. 2015; 290:2321–2333.
Article23. Yamaguchi H, Wang HG. The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene. 2001; 20:7779–7786.
Article24. Xie C, Zhou M, Lin J, Wu Z, Ding S, Luo J, et al. EEF1D promotes glioma proliferation, migration, and invasion through EMT and PI3K/Akt pathway. Biomed Res Int. 2020; 2020:7804706.
Article25. Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012; 4:a011189.
Article26. Quan C, Sun J, Lin Z, Jin T, Dong B, Meng Z, et al. Ezrin promotes pancreatic cancer cell proliferation and invasion through activating the Akt/mTOR pathway and inducing YAP translocation. Cancer Manag Res. 2019; 11:6553–6566.27. Koh SH, Park HH. Neurogenesis in stroke recovery. Transl Stroke Res. 2017; 8:3–13.
Article28. Zhang S, Lachance BB, Moiz B, Jia X. Optimizing stem cell therapy after ischemic brain injury. J Stroke. 2020; 22:286–305.
Article29. Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004; 26:148–165.
Article30. Wei ZZ, Zhang JY, Taylor TM, Gu X, Zhao Y, Wei L. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J Cereb Blood Flow Metab. 2018; 38:404–421.
Article31. Richardsen E, Andersen S, Al-Saad S, Rakaee M, Nordby Y, Pedersen MI, et al. Evaluation of the proliferation marker Ki-67 in a large prostatectomy cohort. PLoS One. 2017; 12:e0186852.
Article32. Lange SS, Mitchell DL, Vasquez KM. High mobility group protein B1 enhances DNA repair and chromatin modification after DNA damage. Proc Natl Acad Sci U S A. 2008; 105:10320–10325.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The individual and combined neuroprotective effects of propofol and ketamine on rat mixed cortical cultures exposed to oxygen-glucose deprivation-reperfusion injury
- The influence of propofol administration time on oxygen-glucose deprivation-reperfusion injury in rat mixed cortical cultures focused on N-Methyl-D-Aspartate (NMDA) receptors
- BMP‑6 Attenuates Oxygen and Glucose Deprivation-Induced Apoptosis in Human Neural Stem Cells through Inhibiting p38 MAPK Signaling Pathway
- The optimal model of reperfusion injury in vitro using H9c2 transformed cardiac myoblasts
- Gene Expression Profile in Microglia following Ischemia-Reperfusion Injury