Korean J Gastroenterol.
2021 Apr;77(4):171-178. 10.4166/kjg.2020.171.
Multicenter, Randomized, Placebo-controlled Trial to Evaluate the Efficacy and Safety of a Controlled-release, Once-daily UIC201609/UIC201610 Combination Therapy for Functional Dyspepsia: Preliminary Study
- Affiliations
-
- 1Department of Internal medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 3Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Digestive Disease Research Institute, Wonkwang University College of Medicine, Iksan, Korea
- 5Department of Gastroenterology, Ajou University School of Medicine, Suwon, Korea
- 6Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2515138
- DOI: http://doi.org/10.4166/kjg.2020.171
Abstract
- Background/Aims
Functional dyspepsia is a disease involving a range of upper gastrointestinal symptoms derived from various pathophysiologies. Tablets containing a combination of rabeprazole and controlled-release (CR) mosapride were recently developed. To investigate a more effective treatment, this trial evaluated the efficacy and safety of UIC201609/UIC201610 as a preliminary study.
Methods
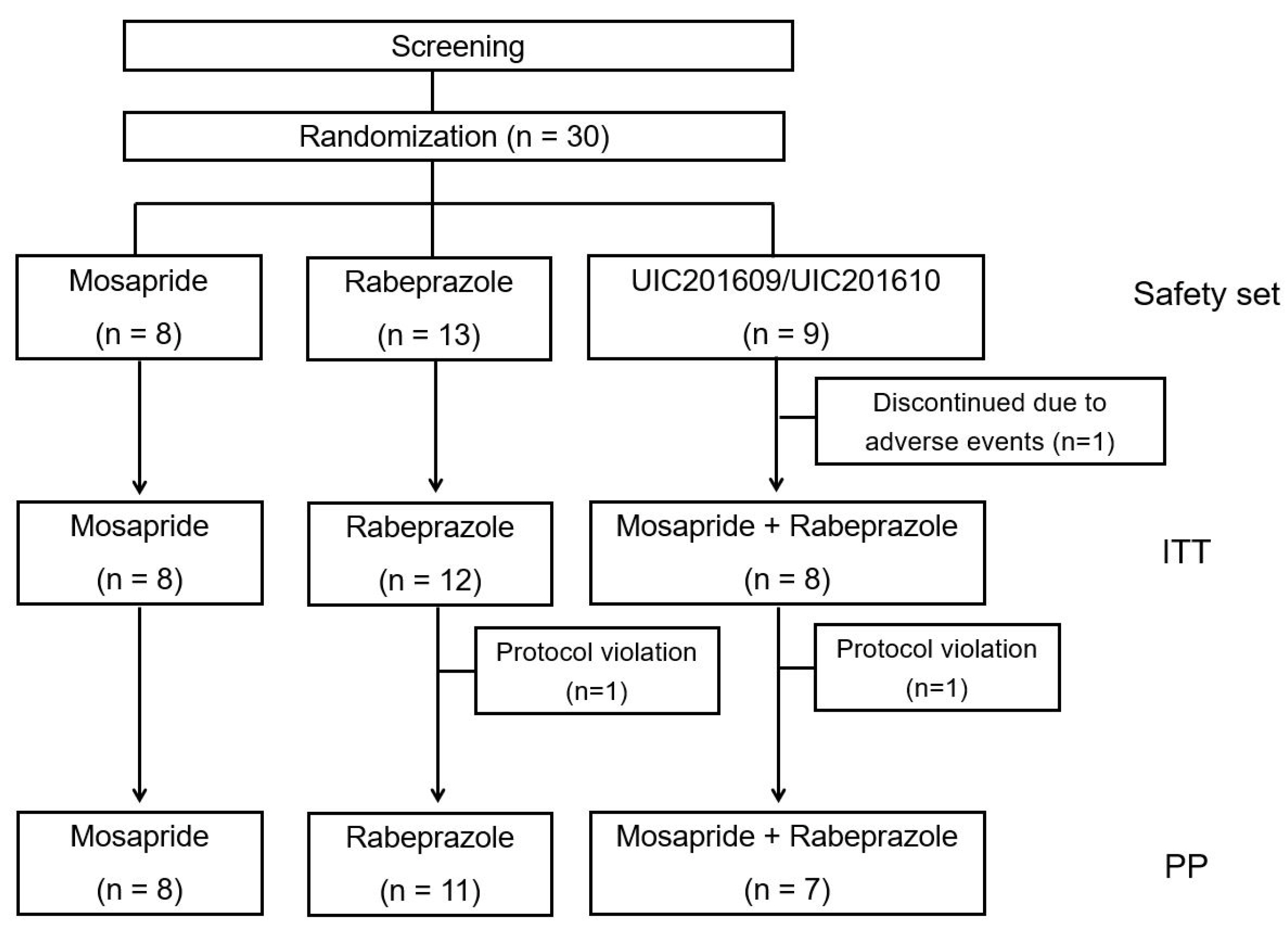
A multicenter, double-blind, randomized study was performed on 30 subjects. UIC201609/UIC201610 (combination of rabeprazole and CR mosapride) was the case group, and the two control groups were rabeprazole 10 mg once a day and mosapride 15 mg CR tablet once a day. As a primary efficacy endpoint of the study, the changes in the total score of eight items of the Nepean Dyspepsia Index-Korean version were analyzed at 2 weeks and 4 weeks. The outcomes regarding safety were collected.
Results
The total symptom score of Nepean Dyspepsia Index-Korean decreased in the rabeprazole single group (29.4±17.1), mosapride CR single group (33.4±15.6), and UIC201609/UIC201610 group (33.4±11.8) at 4 weeks without significant differences. On the other hand, the UIC201609/UIC201610 combination group showed more score reduction of pain in the upper abdomen, burning in the upper abdomen compared to each control group, but it did not reach statistical significance. No difference was found in safety analysis.
Conclusions
UIC201609/UIC201610 once daily showed some improvement in epigastric pain and dyspepsia in patients with functional dyspepsia, but there was no significance. Further study based on the advanced clinical trial design will be needed to confirm the efficacy of UIC201609/UIC201610 combination therapy in the future.
Figure
Cited by 1 articles
-
The Effect of Proton Pump Inhibitor and Prokinetics Combination Therapy for Functional Dyspepsia
Ju Yup Lee
Korean J Gastroenterol. 2021;77(4):149-150. doi: 10.4166/kjg.2021.060.
Reference
-
1. Stanghellini V, Chan FK, Hasler WL, et al. 2016; Gastroduodenal disorders. Gastroenterology. 150:1380–1392. DOI: 10.1053/j.gastro.2016.02.011. PMID: 27147122.
Article2. Kim SE, Park HK, Kim N, et al. 2014; Prevalence and risk factors of functional dyspepsia: a nationwide multicenter prospective study in Korea. J Clin Gastroenterol. 48:e12–e18. DOI: 10.1097/MCG.0b013e31828f4bc9. PMID: 23632355.3. Lee JY, Kim N, Nam RH, In Choi S, Lee JW, Lee DH. 2019; Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 24:e12660. DOI: 10.1111/hel.12660. PMCID: PMC6790945.
Article4. Oh JH, Kwon JG, Jung HK, et al. 2020; Clinical practice guidelines for functional dyspepsia in Korea. J Neurogastroenterol Motil. 26:29–50. DOI: 10.5056/jnm19209. PMID: 31917913. PMCID: PMC6955183.
Article5. Lee JH, Choi KD, Jung HY, et al. 2018; Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. 23:e12463. DOI: 10.1111/hel.12463. PMID: 29345022. PMCID: PMC5900911.6. Kim SE, Kim N, Lee JY, et al. 2018; Prevalence and risk factors of functional dyspepsia in health check-up population: a nationwide multicenter prospective study. J Neurogastroenterol Motil. 24:603–613. DOI: 10.5056/jnm18068. PMID: 29938463. PMCID: PMC6175566.
Article7. Pinto-Sanchez MI, Yuan Y, Bercik P, Moayyedi P. 2017; Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 3:CD011194. DOI: 10.1002/14651858.CD011194.pub3. PMID: 29161458. PMCID: PMC6485982.
Article8. Quigley EM. 2015; Prokinetics in the management of functional gastrointestinal disorders. J Neurogastroenterol Motil. 21:330–336. DOI: 10.5056/jnm15094. PMID: 26130629. PMCID: PMC4496896.
Article9. Pittayanon R, Yuan Y, Bollegala NP, et al. 2019; Prokinetics for functional dyspepsia: a systematic review and meta-analysis of randomized control trials. Am J Gastroenterol. 114:233–243. DOI: 10.1038/s41395-018-0258-6. PMID: 30337705.
Article10. Jones M, Talley NJ. 2009; Minimum clinically important difference for the Nepean Dyspepsia Index, a validated quality of life scale for functional dyspepsia. Am J Gastroenterol. 104:1483–1488. DOI: 10.1038/ajg.2009.136. PMID: 19491862.
Article11. Cho YK, Choi MG, Kim SH, et al. 2004; The effect of mosapride on quality of life in functional dyspepsia. Korean J Gastroenterol. 43:160–167.12. Tack J, Bisschops R, Sarnelli G. 2004; Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 127:1239–1255. DOI: 10.1053/j.gastro.2004.05.030. PMID: 15481001.
Article13. Miwa H, Ghoshal UC, Gonlachanvit S, et al. 2012; Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 18:150–168. DOI: 10.5056/jnm.2012.18.2.150. PMID: 22523724. PMCID: PMC3325300.
Article14. Tack J, Talley NJ, Camilleri M, et al. 2006; Functional gastroduodenal disorders. Gastroenterology. 130:1466–1479. DOI: 10.1053/j.gastro.2005.11.059. PMID: 16678560.
Article15. Yang YJ, Bang CS, Baik GH, et al. 2017; Prokinetics for the treatment of functional dyspepsia: Bayesian network meta-analysis. BMC Gastroenterol. 17:83. DOI: 10.1186/s12876-017-0639-0. PMID: 28651565. PMCID: PMC5485548.
Article16. Shin HW, Kim MJ, Kim JS, Lee MC, Chung SJ. 2009; Levosulpiride-induced movement disorders. Mov Disord. 24:2249–2253. DOI: 10.1002/mds.22805. PMID: 19795476.
Article17. Frommeyer G, Fischer C, Ellermann C, et al. 2017; Severe proarrhythmic potential of the antiemetic agents ondansetron and domperidone. Cardiovasc Toxicol. 17:451–457. DOI: 10.1007/s12012-017-9403-5. PMID: 28185059.
Article18. Jung HK, Lee KJ, Choi MG, et al. 2016; Efficacy of DA-9701 (motilitone) in functional dyspepsia compared to pantoprazole: a multicenter, randomized, double-blind, non-inferiority study. J Neurogastroenterol Motil. 22:254–263. DOI: 10.5056/jnm15178. PMID: 26811504. PMCID: PMC4819864.
Article19. Bang CS, Kim JH, Baik GH, et al. 2015; Mosapride treatment for functional dyspepsia: a meta-analysis. J Gastroenterol Hepatol. 0:28–42. DOI: 10.1111/jgh.12662. PMID: 25041564.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Clidinium/Chlordiazepoxide as an Add-on Therapy in Functional Dyspepsia: A Randomized, Controlled, Trial
- Treatment of Helicobacter pylori infection in functional dyspepsia
- Efficacy and Safety of New Prokinetic Agent Benachio Q Solution(R) in Patients with Postprandial Distress Syndrome Subtype in Functional Dyspepsia: A Single-center, Randomized, Double-blind, Placebo-controlled Pilot Study
- The Efficacy and Safety of GCWB104 (Flos Lonicera Extract) in Functional Dyspepsia: A Single-Center, Randomized, Double-Blind, Placebo-Controlled Study
- Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension