Korean J Gastroenterol.
2015 Jul;66(1):17-26. 10.4166/kjg.2015.66.1.17.
Efficacy and Safety of New Prokinetic Agent Benachio Q Solution(R) in Patients with Postprandial Distress Syndrome Subtype in Functional Dyspepsia: A Single-center, Randomized, Double-blind, Placebo-controlled Pilot Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. nayoungkim49@empas.com
- 2Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2373307
- DOI: http://doi.org/10.4166/kjg.2015.66.1.17
Abstract
- BACKGROUND/AIMS
Functional dyspepsia (FD) is a gastrointestinal disorder in which the patient suffers from chronic abdominal symptoms despite the absence of organic disease. Benachio Q solution (soln.)(R) is a new prokinetic herbal medicine. The aim of the present study is to determine the efficacy and safety of Benachio Q soln.(R) in patients with postprandial distress syndrome (PDS) subtype in FD.
METHODS
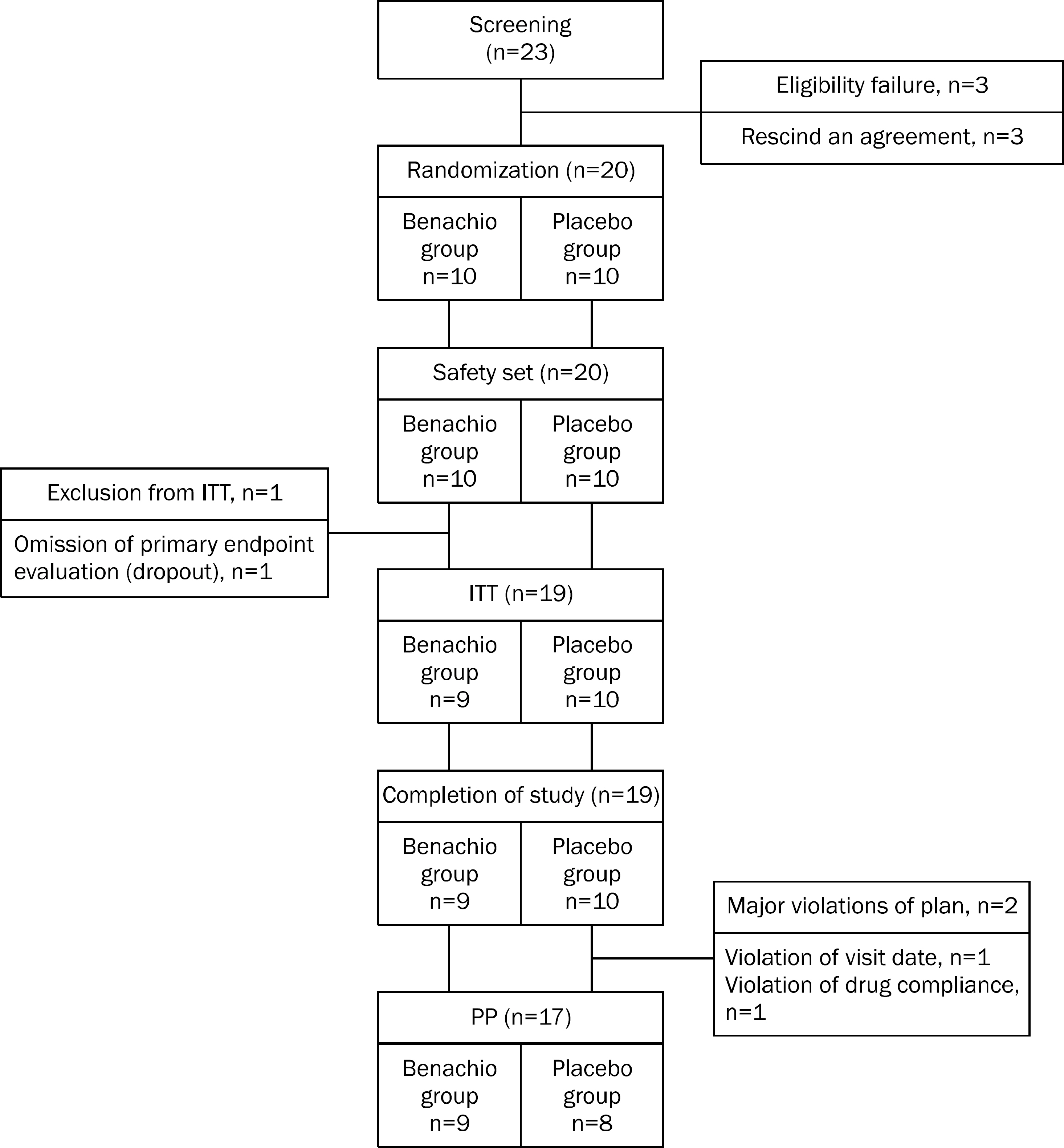
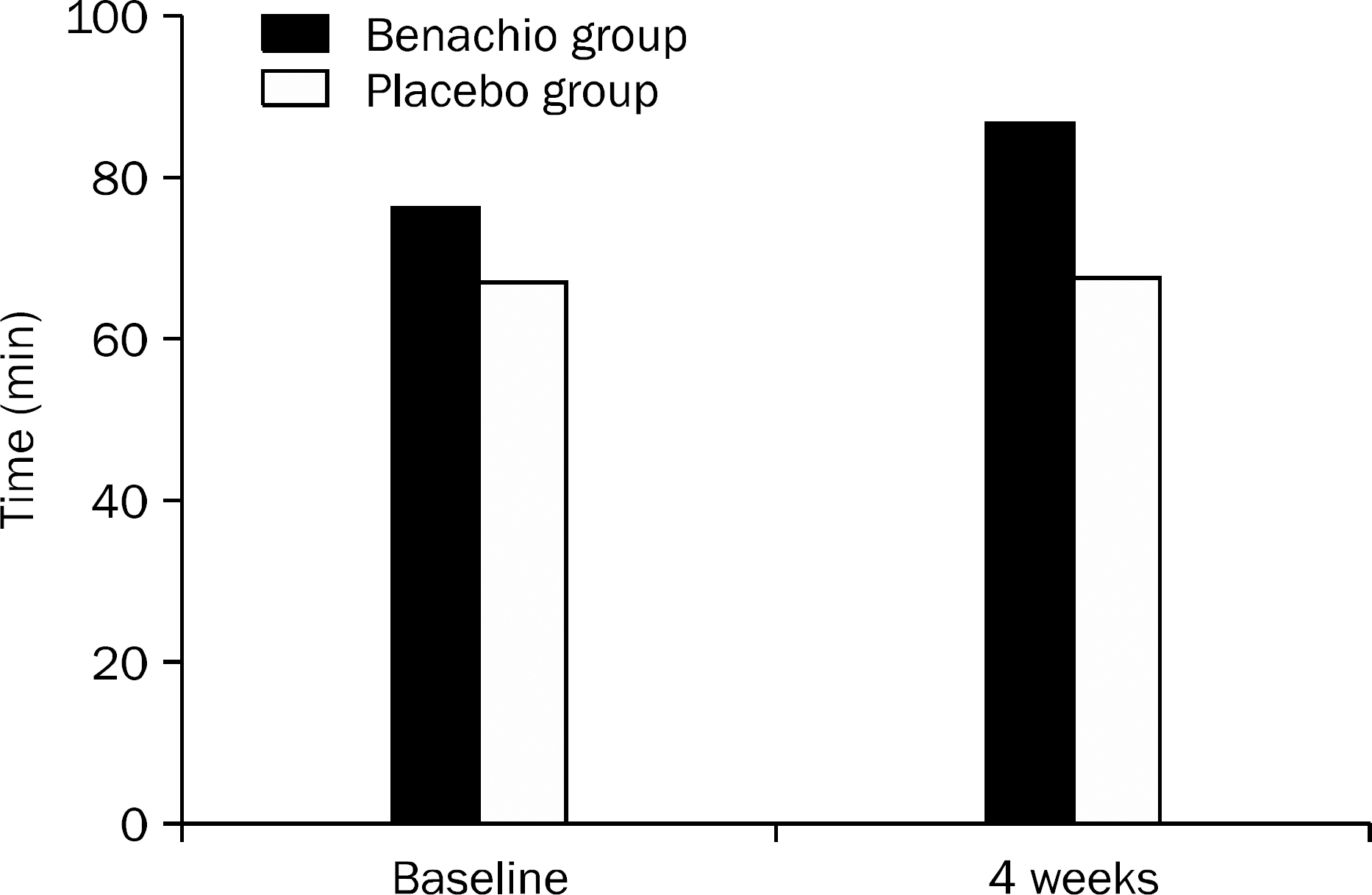
A single-center, randomized, double-blind, placebo-controlled pilot study was performed in 20 patients with PDS. Patients were assigned to receive either Benachio Q soln.(R) or placebo three times a day. After 4 weeks of treatment, the data on response rates, symptoms severity of PDS and gastric emptying time were analyzed to evaluate its efficacy. Adverse events, laboratory tests and vital sign were analyzed to assess its safety.
RESULTS
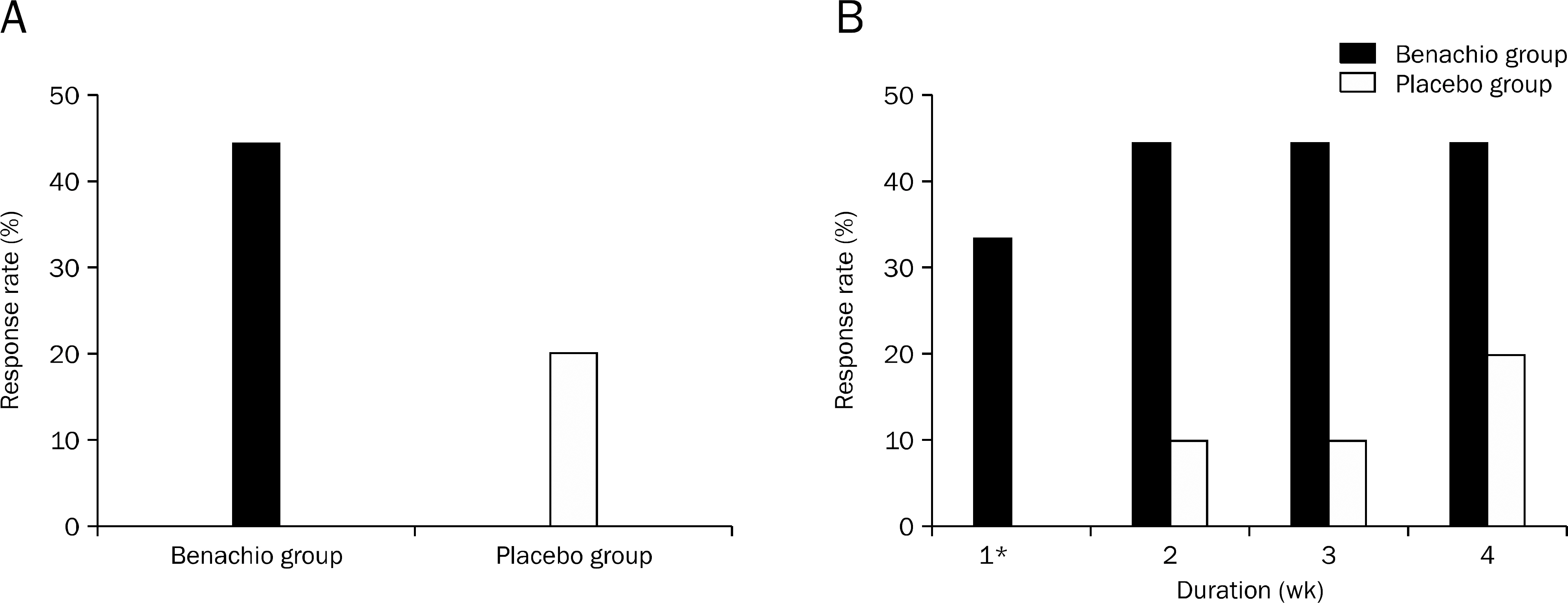
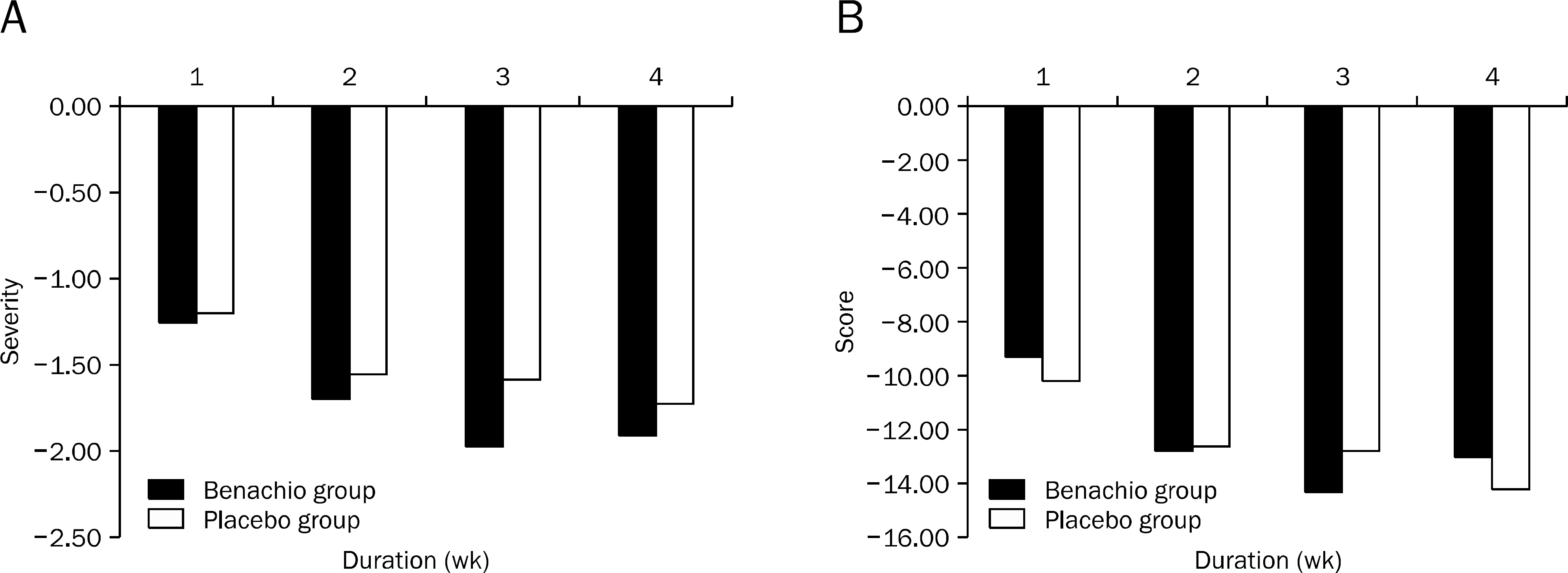
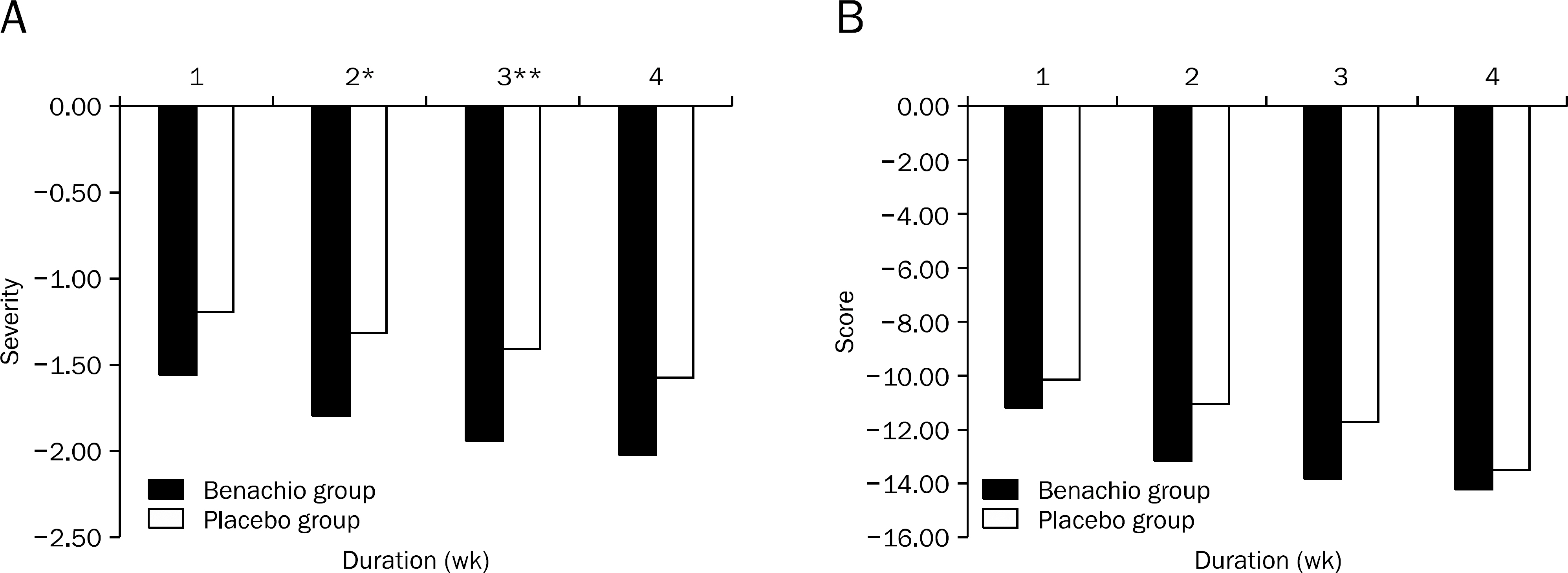
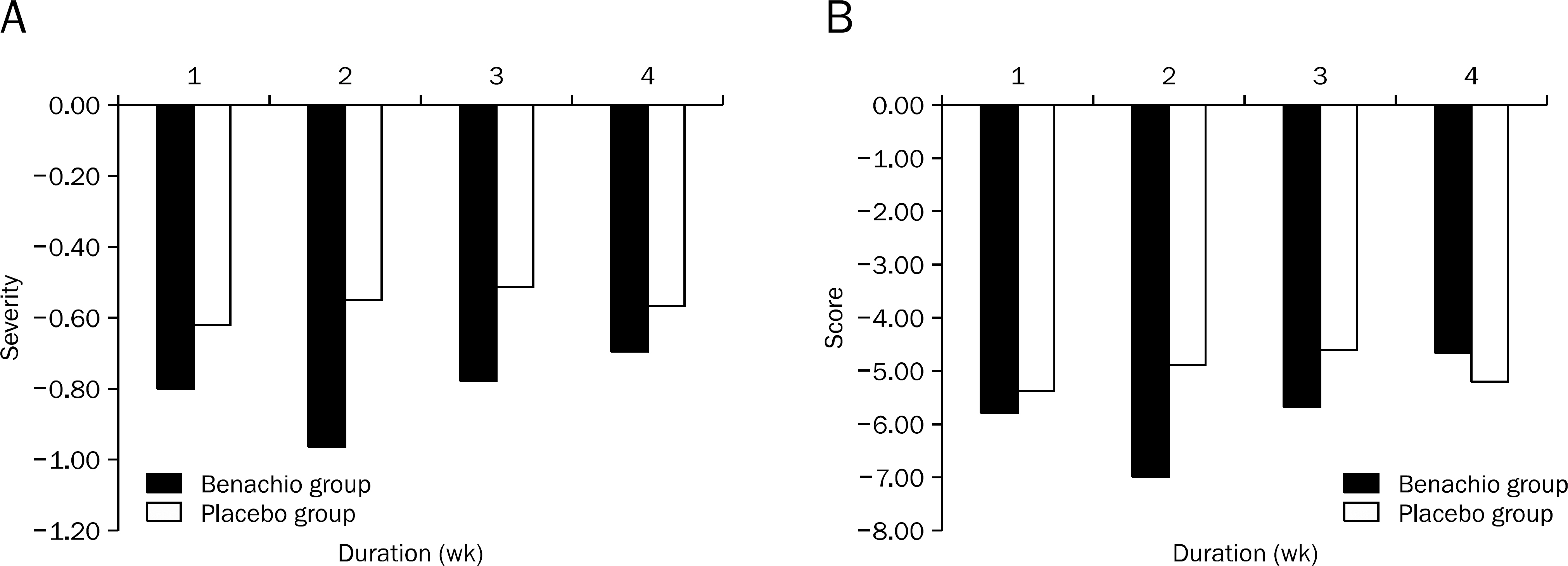
Nine patients were assigned to Benachio group and 10 patients to placebo group. The response rate after 4 weeks was 44.4% and 20.0% in Benachio and placebo group, respectively (p=0.350). The response rate during the first week in Benachio group was better compared to that of placebo group with marginal difference (33.3% vs. 0.0%, p=0.087). Changes of severity score in early satiety on second and third week were -1.8+/-0.6, -1.9+/-0.4 and -1.3+/-0.5, -1.4+/-0.6 in Benachio and placebo group, respectively (p=0.059 vs. p=0.033). No adverse event was observed.
CONCLUSIONS
The new herbal drug, Benachio Q soln.(R) seems to improve the symptoms of PDS subtype in FD and could be used safely. Further larger trial is needed in the future.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Noh YW, Jung HK, Kim SE, Jung SA. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J Neurogastroenterol Motil. 2010; 16:148–156.
Article2. Jones R, Lydeard S. Prevalence of symptoms of dyspepsia in the community. BMJ. 1989; 298:30–32.
Article3. Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ 3rd. Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992; 102:1259–1268.
Article4. Oh KH, Nam Y, Jeong JH, Kim IK, Sohn UD. The effect of DA-9701 on 5-hydroxytryptamine-induced contraction of feline esophageal smooth muscle cells. Molecules. 2014; 19:5135–5149.
Article5. Qin F, Huang X, Ren P. Chinese herbal medicine modified xiaoyao san for functional dyspepsia: metaanalysis of randomized controlled trials. J Gastroenterol Hepatol. 2009; 24:1320–1325.
Article6. Lee HT. Prokinetic activity of ethanolic extracts from dried Citrus unshiu peels in mice. J Life Sci. 2014; 24:260–265.
Article7. Lyu JH, Lee HT. Effects of dried Citrus unshiu peels on gastrointestinal motility in rodents. Arch Pharm Res. 2013; 36:641–648.
Article8. Noh HJ, Hwang D, Lee ES, et al. Anti-inflammatory activity of a new cyclic peptide, citrusin XI, isolated from the fruits of Citrus unshiu. J Ethnopharmacol. 2015; 163:106–112.
Article9. Hu ML, Rayner CK, Wu KL, et al. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J Gastroenterol. 2011; 17:105–110.
Article10. Ernst E, Pittler MH. Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br J Anaesth. 2000; 84:367–371.
Article11. Yamahara J, Huang QR, Li YH, Xu L, Fujimura H. Gastrointestinal motility enhancing effect of ginger and its active constituents. Chem Pharm Bull (Tokyo). 1990; 38:430–431.
Article12. Hlebowicz J, Darwiche G, Björgell O, Almér LO. Effect of cinna-mon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. 2007; 85:1552–1556.
Article13. Kubo M, Ma S, Wu J, Matsuda H. Anti-inflammatory activities of 70% methanolic extract from Cinnamomi Cortex. Biol Pharm Bull. 1996; 19:1041–1045.
Article14. Nakai Y, Kido T, Hashimoto K, et al. Effect of the rhizomes of Atractylodes lancea and its constituents on the delay of gastric emptying. J Ethnopharmacol. 2003; 84:51–55.
Article15. Kimura Y, Sumiyoshi M. Effects of an Atractylodes lancea rhi-zome extract and a volatile component β-eudesmol on gastrointestinal motility in mice. J Ethnopharmacol. 2012; 141:530–536.
Article16. Yang IJ, Yu HY, Lee DU, Shin HM. Anti-inflammatory effects of the fruits of Foeniculum vulgare in lipopolysaccharide stimulated macrophages. J Life Sci. 2014; 24:981–987.17. Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012; 61:821–828.
Article18. Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, Forman D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006; (4):CD001960.
Article19. Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a metaanalysis. Am J Gastroenterol. 2001; 96:689–696.20. Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006; 354:832–840.
Article21. Talley NJ. Subdividing functional dyspepsia: a paradigm shift? Gut. 2008; 57:1487–1489.
Article22. Hallerbäck BI, Bommelaer G, Bredberg E, et al. Dose finding study of mosapride in functional dyspepsia: a placebo-controlled, randomized study. Aliment Pharmacol Ther. 2002; 16:959–967.
Article23. Cho YK, Choi MG, Kim SH, et al. The effect of mosapride on quality of life in functional dyspepsia. Korean J Gastroenterol. 2004; 43:160–167.24. Holtmann G, Talley NJ. Herbal medicines for the treatment of functional and inflammatory bowel disorders. Clin Gastroenterol Hepatol. 2015; 13:422–432.25. Kwon YS, Son M. DA-9701: a new multi-acting drug for the treatment of functional dyspepsia. Biomol Ther (Seoul). 2013; 21:181–189.
Article26. Park CH, Kim HS, Lee SK. Effects of the new prokinetic agent DA-9701 formulated with corydalis tuber and pharbitis seed in patients with minimal change esophagitis: a bicenter, randomized, double blind, placebo-controlled study. J Neurogastroenterol Motil. 2014; 20:338–346.
Article27. Min YW, Ko EJ, Lee JY, et al. Nitrergic pathway is the major mechanism for the effect of DA-9701 on the rat gastric fundus relaxation. J Neurogastroenterol Motil. 2014; 20:318–325.
Article28. Holtmann G, Adam B, Vinson B. Evidence-based medicine and phytotherapy for functional dyspepsia and irritable bowel syndrome: a systematic analysis of evidence for the herbal preparation Iberogast. Wien Med Wochenschr. 2004; 154:528–534.
Article29. Suzuki H, Matsuzaki J, Fukushima Y, et al. Rikkunshito study group. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia–a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil. 2014; 26:950–961.
Article30. Ottillinger B, Storr M, Malfertheiner P, Allescher HD. STW 5 (Iberogast®)–a safe and effective standard in the treatment of functional gastrointestinal disorders. Wien Med Wochenschr. 2013; 163:65–72.
Article31. von Arnim U, Peitz U, Vinson B, Gundermann KJ, Malfertheiner P. STW 5, a phytopharmacon for patients with functional dyspepsia: results of a multicenter, placebo-controlled double-blind study. Am J Gastroenterol. 2007; 102:1268–1275.
Article32. Jung W, Kwon S, Im J, et al. Influence of herbal complexes containing licorice on potassium levels: a retrospective study. Evid Based Complement Alternat Med. 2014. DOI: doi:10.1155/2014/970385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Relationship between Obstructive Sleep Apnea and Functional Dyspepsia
- Clinical Trial: Efficacy of Mosapride Controlledrelease and Nortriptyline in Patients With Functional Dyspepsia: A Multicenter, Double-placebo, Double-blinded, Randomized Controlled, Parallel Clinical Study
- The Efficacy and Safety of GCWB104 (Flos Lonicera Extract) in Functional Dyspepsia: A Single-Center, Randomized, Double-Blind, Placebo-Controlled Study
- The Efficacy and Safety of NOVAponin (Dolichos lablab Linne Extract Powder) in Mild Functional Dyspepsia: A Single-center, Randomized, Double-Blind, Placebo-controlled Study
- Probiotic Supplementation for Treatment of Helicobacter pylori Infection: A Double-Blind Randomized Clinical Trial