Cancer Res Treat.
2021 Apr;53(2):378-388. 10.4143/crt.2020.756.
Atypical Teratoid/Rhabdoid Tumor of the Central Nervous System in Children under the Age of 3 Years
- Affiliations
-
- 1Department of Pediatrics, Center for Pediatric Cancer, National Cancer Center, Goyang, Korea
- 2Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Radiation Oncology, National Cancer Center, Goyang, Korea
- 4Neuro-Oncology Clinic, Center for Pediatric Cancer, National Cancer Center, Goyang, Korea
- 5Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Radiation Oncology, Yonsei University College of Medicine, Seoul, Korea
- 7Department of Pediatrics, Seoul National University Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 8Department of Neurosurgery, Seoul National University College of Medicine, Seoul National University Cancer Research Institute, Seoul, Korea
- 9Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 10Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 11Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 12Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 13Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 14Department of Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 15Department of Pediatrics, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Gwangju, Korea
- 16Department of Neurosurgery, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- 17Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 18Department of Neurosurgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2514920
- DOI: http://doi.org/10.4143/crt.2020.756
Abstract
- Purpose
Atypical teratoid/rhabdoid tumor (ATRT) is a highly aggressive malignancy with peak incidence in children aged less than 3 years. Standard treatment for central nervous system ATRT in children under the age of 3 years have not been established yet. The objective of this study was to analyze characteristics and clinical outcomes of ATRT in children aged less than 3 years.
Materials and Methods
A search of medical records from seven centers was performed between January 2005 and December 2016.
Results
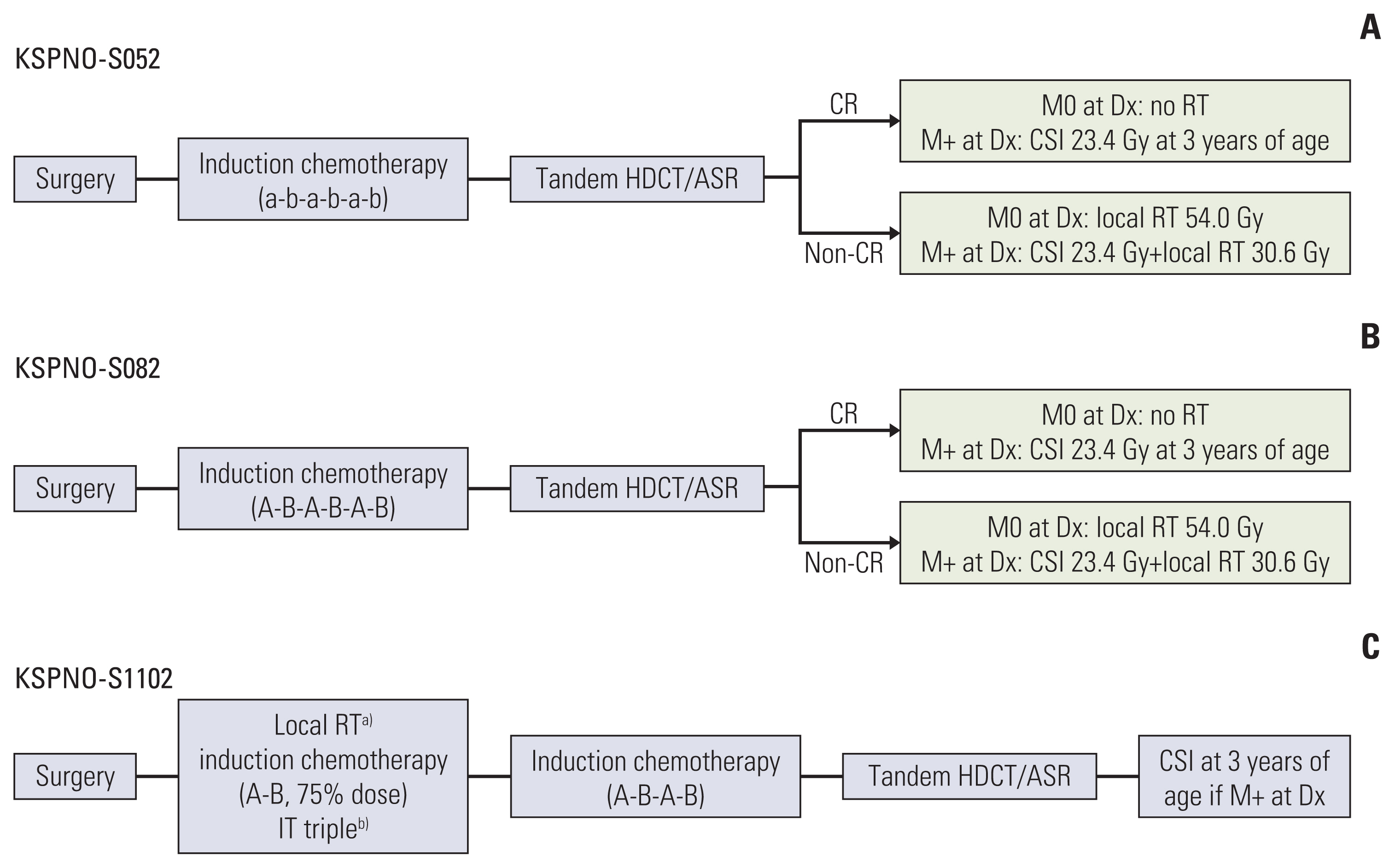
Forty-three patients were enrolled. With a median follow-up of 90 months, 27 patients (64.3%) showed at least one episode of disease progression (PD). The first date of PD was at 160 days after diagnosis. The 1- and 3-year progression-free survivals (PFS) were 51.2% and 28.5%, respectively. The 1- and 3-year overall survivals were 61.9% and 38.1%, respectively. The 3-year PFS was improved from 0% in pre-2011 to 47.4% in post-2011. Excluding one patient who did not receive any further therapy after surgery, 27 patients died due to PD (n=21), treatment-related toxicity (n=5), or unknown cause (n=1). In univariate analysis, factors associated with higher 3-year PFS were no metastases, diagnosis after 2011, early adjuvant radiotherapy, and high-dose chemotherapy (HDCT). In multivariate analysis, the use of HDCT and adjuvant radiotherapy remained significant prognostic factors for PFS (both p < 0.01).
Conclusion
Aggressive therapy including early adjuvant radiotherapy and HDCT could be considered to improve outcomes of ATRT in children under the age of 3 years.
Keyword
Figure
Cited by 1 articles
-
Survival and Malignant Transformation of Pineal Parenchymal Tumors: A 30-Year Retrospective Analysis in a Single-Institution
Tae-Hwan Park, Seung-Ki Kim, Ji Hoon Phi, Chul-Kee Park, Yong Hwy Kim, Sun Ha Paek, Chang-Hyun Lee, Sung-Hye Park, Eun Jung Koh
Brain Tumor Res Treat. 2023;11(4):254-265. doi: 10.14791/btrt.2023.0033.
Reference
-
References
1. Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, et al. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004; 22:2877–84.
Article2. Tekautz TM, Fuller CE, Blaney S, Fouladi M, Broniscer A, Merchant TE, et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005; 23:1491–9.
Article3. Woehrer A, Slavc I, Waldhoer T, Heinzl H, Zielonke N, Czech T, et al. Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian Brain Tumor Registry, 1996–2006. Cancer. 2010; 116:5725–32.4. Cohen BH, Geyer JR, Miller DC, Curran JG, Zhou T, Holmes E, et al. Pilot study of intensive chemotherapy with peripheral hematopoietic cell support for children less than 3 years of age with malignant brain tumors, the CCG-99703 phase I/II study: a report from the Children’s Oncology Group. Pediatr Neurol. 2015; 53:31–46.5. Burger PC, Yu IT, Tihan T, Friedman HS, Strother DR, Kepner JL, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a Pediatric Oncology Group study. Am J Surg Pathol. 1998; 22:1083–92.6. Weiss E, Behring B, Behnke J, Christen HJ, Pekrun A, Hess CF. Treatment of primary malignant rhabdoid tumor of the brain: report of three cases and review of the literature. Int J Radiat Oncol Biol Phys. 1998; 41:1013–9.
Article7. Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004; 5:399–408.
Article8. Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008; 51:235–40.
Article9. Pai Panandiker AS, Merchant TE, Beltran C, Wu S, Sharma S, Boop FA, et al. Sequencing of local therapy affects the pattern of treatment failure and survival in children with atypical teratoid rhabdoid tumors of the central nervous system. Int J Radiat Oncol Biol Phys. 2012; 82:1756–63.
Article10. Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009; 27:385–9.11. Chang CH, Housepian EM, Herbert C Jr. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969; 93:1351–9.
Article12. Rosenfeld A, Kletzel M, Duerst R, Jacobsohn D, Haut P, Weinstein J, et al. A phase II prospective study of sequential myeloablative chemotherapy with hematopoietic stem cell rescue for the treatment of selected high risk and recurrent central nervous system tumors. J Neurooncol. 2010; 97:247–55.
Article13. Chen YW, Wong TT, Ho DM, Huang PI, Chang KP, Shiau CY, et al. Impact of radiotherapy for pediatric CNS atypical teratoid/rhabdoid tumor (single institute experience). Int J Radiat Oncol Biol Phys. 2006; 64:1038–43.
Article14. Lee JY, Kim IK, Phi JH, Wang KC, Cho BK, Park SH, et al. Atypical teratoid/rhabdoid tumors: the need for more active therapeutic measures in younger patients. J Neurooncol. 2012; 107:413–9.
Article15. Sung KW, Lim DH, Yi ES, Choi YB, Lee JW, Yoo KH, et al. Tandem high-dose chemotherapy and autologous stem cell transplantation for atypical teratoid/rhabdoid tumor. Cancer Res Treat. 2016; 48:1408–19.
Article16. Zaky W, Dhall G, Ji L, Haley K, Allen J, Atlas M, et al. Intensive induction chemotherapy followed by myeloablative chemotherapy with autologous hematopoietic progenitor cell rescue for young children newly-diagnosed with central nervous system atypical teratoid/rhabdoid tumors: the Head Start III experience. Pediatr Blood Cancer. 2014; 61:95–101.
Article17. Fossey M, Li H, Afzal S, Carret AS, Eisenstat DD, Fleming A, et al. Atypical teratoid rhabdoid tumor in the first year of life: the Canadian ATRT registry experience and review of the literature. J Neurooncol. 2017; 132:155–62.
Article18. Reddy AT, Strother DR, Judkins AR, Burger PC, Pollack IF, Krailo MD, et al. Efficacy of high-dose chemotherapy and three-dimensional conformal radiation for atypical teratoid/ rhabdoid tumor: a report from the Children’s Oncology Group Trial ACNS0333. J Clin Oncol. 2020; 38:1175–85.19. Richardson EA, Ho B, Huang A. Atypical teratoid rhabdoid tumour: from tumours to therapies. J Korean Neurosurg Soc. 2018; 61:302–11.20. Sung KW, Lee SH, Yoo KH, Jung HL, Cho EJ, Koo HH, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007; 40:37–45.
Article21. Ginn KF, Gajjar A. Atypical teratoid rhabdoid tumor: current therapy and future directions. Front Oncol. 2012; 2:114.
Article22. Lafay-Cousin L, Hawkins C, Carret AS, Johnston D, Zelcer S, Wilson B, et al. Central nervous system atypical teratoid rhabdoid tumours: the Canadian Paediatric Brain Tumour Consortium experience. Eur J Cancer. 2012; 48:353–9.
Article23. Slavc I, Chocholous M, Leiss U, Haberler C, Peyrl A, Azizi AA, et al. Atypical teratoid rhabdoid tumor: improved long-term survival with an intensive multimodal therapy and delayed radiotherapy. The Medical University of Vienna Experience 1992–2012. Cancer Med. 2014; 3:91–100.
Article24. Singh A, Lun X, Jayanthan A, Obaid H, Ruan Y, Strother D, et al. Profiling pathway-specific novel therapeutics in preclinical assessment for central nervous system atypical teratoid rhabdoid tumors (CNS ATRT): favorable activity of targeting EGFR-ErbB2 signaling with lapatinib. Mol Oncol. 2013; 7:497–512.25. Venneti S, Le P, Martinez D, Eaton KW, Shyam N, Jordan-Sciutto KL, et al. p16INK4A and p14ARF tumor suppressor pathways are deregulated in malignant rhabdoid tumors. J Neuropathol Exp Neurol. 2011; 70:596–609.26. Athale UH, Duckworth J, Odame I, Barr R. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediatr Hematol Oncol. 2009; 31:651–63.27. Lafay-Cousin L, Fay-McClymont T, Johnston D, Fryer C, Scheinemann K, Fleming A, et al. Neurocognitive evaluation of long term survivors of atypical teratoid rhabdoid tumors (ATRT): the Canadian registry experience. Pediatr Blood Cancer. 2015; 62:1265–9.
Article28. Duffner PK, Cohen ME. Long-term consequences of CNS treatment for childhood cancer, Part II: Clinical consequences. Pediatr Neurol. 1991; 7:237–42.
Article29. Hasan A, Palumbo M, Atkinson J, Carret AS, Farmer JP, Montes J, et al. Treatment-related morbidity in atypical teratoid/rhabdoid tumor: multifocal necrotizing leukoencephalopathy. Pediatr Neurosurg. 2011; 47:7–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imaging Findings of Atypical Teratoid/Rhabdoid Tumor of Infancy & Childhood in CNS: Report of Two Cases
- Atypical Teratoid Rhabdoid Tumors in Adult Patient with Multiple Lesions
- A Case of Central Nervous System Atypical Teratoid/Rhabdoid Tumor of The 4th Ventricle : A Highly Malignant Tumor in Infancy and Childhood Frequently Mistaken for Medulloblastoma
- A Case of Atypical Teratoid/Rhabdoid Tumor Arising from the Supratentorial Area
- Atypical Teratoid/Rhabdoid Tumor in Central Nervous System: Report of 2 Cases