Ann Lab Med.
2021 May;41(3):268-276. 10.3343/alm.2021.41.3.268.
Immunosuppressive Drug Measurement by Liquid Chromatography Coupled to Tandem Mass Spectrometry: Interlaboratory Comparison in the Korean Clinical Laboratories
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine, Ulsan University Hospital, Ulsan, Korea
- 2Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Laboratory Medicine, Seoul National University Hospital and College of Medicine, Seoul, Korea
- 6Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 7Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea
- 8Seoul Clinical Laboratories, Yongin, Korea
- 9Green Cross Laboratories, Yongin, Korea
- 10Department of Laboratory Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 11Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- KMID: 2512684
- DOI: http://doi.org/10.3343/alm.2021.41.3.268
Abstract
- Background
Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) is increasingly used for immunosuppressive drug tests. However, most LC-MS/MS tests are laboratory-developed and their agreement is unknown in different Korean laboratories. This interlaboratory comparison study evaluated test reproducibility and identified potential error sources.
Methods
Test samples containing three concentrations of tacrolimus, sirolimus, everolimus, cyclosporine, and mycophenolic acid were prepared by pooling surplus samples from patients undergoing routine therapeutic drug monitoring and tested in duplicate in the participating 10 clinical laboratories. Reconstitution and storage experiments were conducted for the commonly used commercial calibrator set. The robust estimators of reproducibility parameters were calculated. Spearman’s rank correlation coefficient (rho, ρ) was used to evaluate the correlation between drugs. Multiple linear regression was used to determine whether the experimental conditions alter the calibration curves.
Results
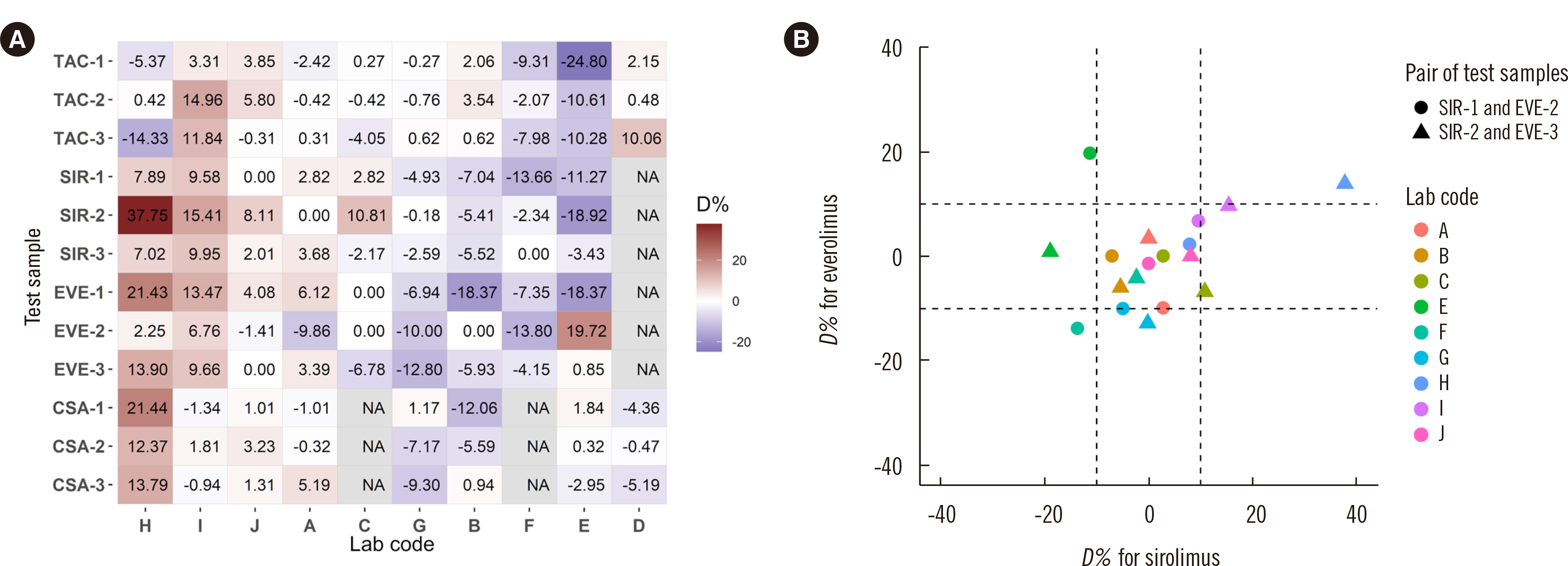
The reproducibility coefficient of variation exceeded 10% only for sirolimus concentrations 1 and 2 (10.8% and 12.5%, respectively) and everolimus concentrations 1 and 2 (12.3% and 11.4%, respectively). The percent difference values showed weak correlations between sirolimus and everolimus (ρ = 0.334, P = 0.175). The everolimus calibration curve slope was significantly altered after reconstitution following prolonged 5°C storage (P = 0.015 for 14 days; P = 0.025 for 28 days); the expected differences at 6 ng/mL were 0.598% for 14 days and 0.384% for 28 days.
Conclusions
LC-MS/MS test reproducibility for immunosuppressive drugs seems to be good in the Korean clinical laboratories. Continuous efforts are required to achieve test standardization and harmonization, especially for sirolimus and everolimus.
Keyword
Figure
Cited by 2 articles
-
Back to the Basics of Liquid Chromatography-Mass Spectrometry
Young Jin Kim, Soo-Youn Lee, Mina Hur
Ann Lab Med. 2022;42(2):119-120. doi: 10.3343/alm.2022.42.2.119.Evaluation of Vancomycin Area Under the Concentration–Time Curve Predictive Performance Using Bayesian Modeling Software With and Without Peak Concentration: An Academic Hospital Experience for Adult Patients Without Renal Impairment
Hyun-Ki Kim, Tae-Dong Jeong
Ann Lab Med. 2023;43(6):554-564. doi: 10.3343/alm.2023.43.6.554.
Reference
-
1. McShane AJ, Bunch DR, Wang S. 2016; Therapeutic drug monitoring of immunosuppressants by liquid chromatography-mass spectrometry. Clin Chim Acta. 454:1–5. DOI: 10.1016/j.cca.2015.12.027. PMID: 26721314.
Article2. Zhang Y, Zhang R. 2018; Recent advances in analytical methods for the therapeutic drug monitoring of immunosuppressive drugs. Drug Test Anal. 10:81–94. DOI: 10.1002/dta.2290. PMID: 28851030.
Article3. Khan S, Khan S, Baboota S, Ali J. 2015; Immunosuppressive drug therapy-biopharmaceutical challenges and remedies. Expert Opin Drug Deliv. 12:1333–49. DOI: 10.1517/17425247.2015.1005072. PMID: 25642742.4. Mohammadpour N, Elyasi S, Vahdati N, Mohammadpour AH, Shamsara J. 2011; A review on therapeutic drug monitoring of immunosuppressant drugs. Iran J Basic Med Sci. 14:485–98. PMID: 23493821. PMCID: PMC3586862.5. Bentata Y. 2020; Mycophenolates: the latest modern and potent immunosuppressive drugs in adult kidney transplantation: what we should know about them? Artif Organs. 44:561–76. DOI: 10.1111/aor.13623. PMID: 31879962.
Article6. Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, et al. 2010; Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol. 5:341–58. DOI: 10.2215/CJN.07111009. PMID: 20056756.
Article7. Annesley TM, McKeown DA, Holt DW, Mussell C, Champarnaud E, Harter L, et al. 2013; Standardization of LC-MS for therapeutic drug monitoring of tacrolimus. Clin Chem. 59:1630–7. DOI: 10.1373/clinchem.2013.209114. PMID: 23902823.
Article8. CAP. 2018. Immunosuppressive drugs. CSM-A 2018; participant summary. College of American Pathologists;Northfield, IL:9. KEQAS. 2018. Immunosuppressants therapeutic drug monitoring 2018; summary report. Korean Association of External Quality Assessment Service.10. Berlinger B, Harper M. 2018; Interlaboratory comparison for the determination of the soluble fraction of metals in welding fume samples. J Occup Environ Hyg. 15:152–6. DOI: 10.1080/15459624.2017.1395961. PMID: 29157175.
Article11. Christians U, Vinks AA, Langman LJ, Clarke W, Wallemacq P, van Gelder T, et al. 2015; Impact of laboratory practices on interlaboratory variability in therapeutic drug monitoring of immunosuppressive drugs. Ther Drug Monit. 37:718–24. DOI: 10.1097/FTD.0000000000000205. PMID: 26291980.
Article12. Pitkälä A, Gindonis V, Wallin H, Honkanen-Buzalski T. 2005; Interlaboratory proficiency testing as a tool for improving performance in laboratories diagnosing bovine mastitis. J Dairy Sci. 88:553–9. DOI: 10.3168/jds.S0022-0302(05)72717-X. PMID: 15653520.
Article13. Jeong TD, Lee H, Lee K, Yun YM. 2019; Accuracy-based proficiency testing of creatinine measurement: 7 Years' Experience in Korea. J Lab Med Qual Assur. 41:13–23. DOI: 10.15263/jlmqa.2019.41.1.13.
Article14. Chae H, Cho SE, Park HD, Chun S, Lee YW, Yun YM, et al. 2019; Use of liquid chromatography-tandem mass spectrometry for clinical testing in Korean laboratories: a questionnaire survey. Ann Lab Med. 39:447–53. DOI: 10.3343/alm.2019.39.5.447. PMID: 31037863. PMCID: PMC6502944.
Article15. Volodarsky ET, Warsza ZL, Kosheva L, Idźkowski A. 2015; Robust algorithm S to assess the precision of interlaboratory measurements. Meas Autom Monit. 61:111–4.16. ISO. 2015. Statistical methods for use in proficiency testing by interlaboratory comparison. ISO 13528:2015. International Organization for Standardization;Geneva:17. Honour JW. 2011; Development and validation of a quantitative assay based on tandem mass spectrometry. Ann Clin Biochem. 48:97–111. DOI: 10.1258/acb.2010.010176. PMID: 21303874.
Article18. CAP. 2018. EV-A 2018; participant summary. College of American Pathologists;Northfield, IL:19. CAP. 2018. EV-B 2018; participant summar. College of American Pathologists;Northfield, IL:20. CAP. 2018. CSM-B 2018; participant summary. College of American Pathologists;Northfield, IL:21. Chromsystems. 2018. 6PLUS1 multilevel calibrator set, immunosuppressants. 28039. Chromsystems Instruments and Chemicals GmbH;Gräfelfing:22. Carter GD, Jones JC. 2009; Use of a common standard improves the performance of liquid chromatography-tandem mass spectrometry methods for serum 25-hydroxyvitamin-D. Ann Clin Biochem. 46:79–81. DOI: 10.1258/acb.2008.008135. PMID: 19103962.
Article23. Levine DM, Maine GT, Armbruster DA, Mussell C, Buchholz C, O'Connor G, et al. 2011; The need for standardization of tacrolimus assays. Clin Chem. 57:1739–47. DOI: 10.1373/clinchem.2011.172080. PMID: 21998339.
Article24. Valbuena H, Shipkova M, Kliesch SM, Müller S, Wieland E. 2016; Comparing the effect of isotopically labeled or structural analog internal standards on the performance of a LC-MS/MS method to determine ciclosporin A, everolimus, sirolimus and tacrolimus in whole blood. Clin Chem Lab Med. 54:437–46. DOI: 10.1515/cclm-2015-0519. PMID: 26351941.
Article25. Zhao Y, Liu G, Shen JX, Aubry AF. 2014; Reasons for calibration standard curve slope variation in LC-MS assays and how to address it. Bioanalysis. 6:1439–43. DOI: 10.4155/bio.14.71. PMID: 25046045.
Article26. Schniedewind B, Meyer EJ, Christians U. 2020; Long-term performance of laboratory-developed liquid chromatography-tandem mass spectrometry tests and a Food and Drug Administration-approved immunoassay for the therapeutic drug monitoring of everolimus. Ther Drug Monit. 42:421–6. DOI: 10.1097/FTD.0000000000000706. PMID: 32427781.
Article27. Wong SK. 2011; Performance evaluation for proficiency testing with a limited number of participants. Accred Qual Assur. 16:539. DOI: 10.1007/s00769-011-0816-8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recommendations for the Use of Liquid Chromatography-Mass Spectrometry in the Clinical Laboratory: Part I. Implementation and Management
- Recommendations for Liquid Chromatography-Mass Spectrometry in the Clinical Laboratory: Part III. Quality Assurance
- Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits
- A Liquid Chromatography-Tandem Mass Spectrometry Method for Simultaneously Determining Meropenem and Linezolid in Blood and Cerebrospinal Fluid
- Development and validation of analytical method for the determination of radotinib in human plasma using liquid chromatography-tandem mass spectrometry