Korean J Physiol Pharmacol.
2017 Jan;21(1):45-54. 10.4196/kjpp.2017.21.1.45.
Metabolism and excretion of novel pulmonary-targeting docetaxel liposome in rabbits
- Affiliations
-
- 1Pharmacy College, Chongqing Medical University, Chongqing 400016, China. Yuyu_CQMU@outlook.com
- KMID: 2371067
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.45
Abstract
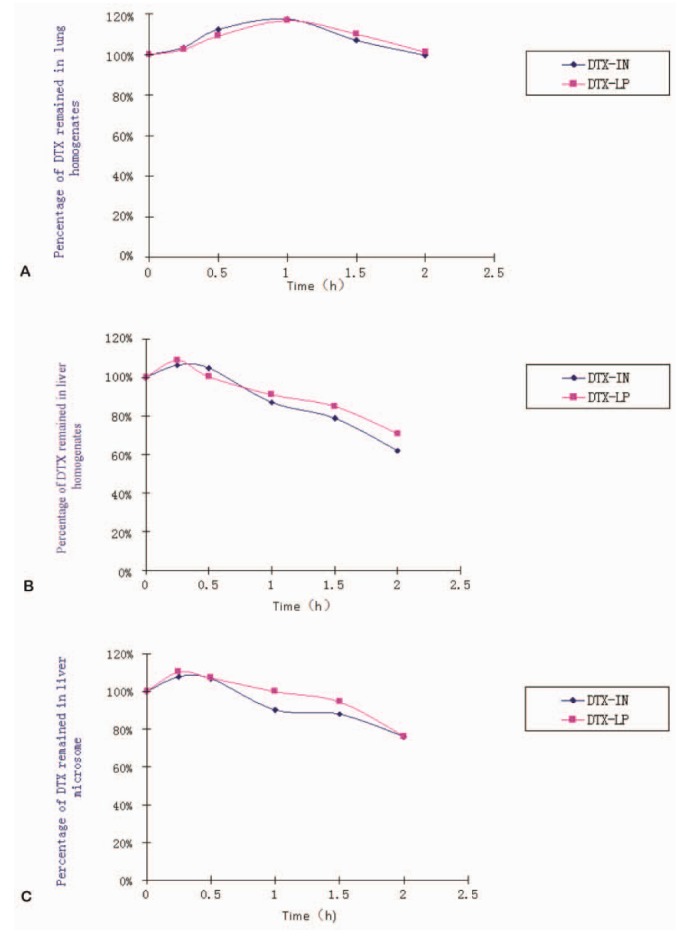
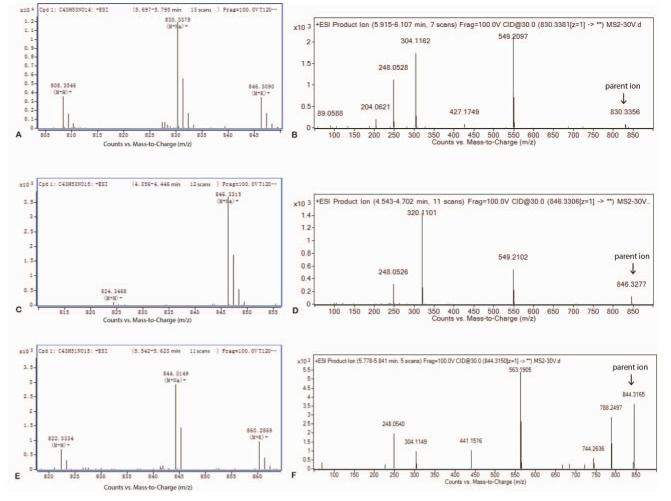
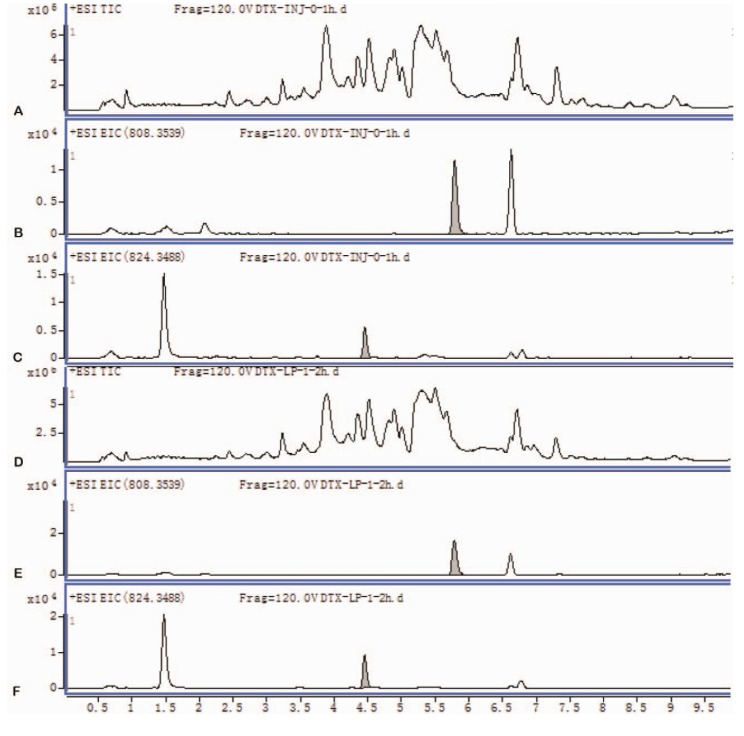
- Our study aims to determine the metabolism and excretion of novel pulmonary-targeting docetaxel liposome (DTX-LP) using the in vitro and in vivo animal experimental models. The metabolism and excretion of DTX-LP and intravenous DTX (DTX-IN) in New Zealand rabbits were determined with ultraperformance liquid chromatography tandem mass spectrometry. We found DTX-LP and DTX-IN were similarly degraded in vitro by liver homogenates and microsomes, but not metabolized by lung homogenates. Ultra-performance liquid chromatography tandem mass spectrometry identified two shared DTX metabolites. The unconfirmed metabolite M(un) differed structurally from all DTX metabolites identified to date. DTX-LP likewise had a similar in vivo metabolism to DTX-IN. Conversely, DTX-LP showed significantly diminished excretion in rabbit feces or urine, approximately halving the cumulative excretion rates compared to DTX-IN. Liposomal delivery of DTX did not alter the in vitro or in vivo drug metabolism. Delayed excretion of pulmonary-targeting DTX-LP may greatly enhance the therapeutic efficacy and reduce the systemic toxicity in the chemotherapy of non-small cell lung cancer. The identification of M(un) may further suggest an alternative species-specific metabolic pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013; 382:709–719. PMID: 23972814.
Article2. Stanisic S, Bischoff HG, Heigener DF, Vergnenègre A, de Castro Carpeño J, Chouaid C, Walzer S, Mueller E, Schmidt E. Societal cost savings through bevacizumab-based treatment in non-small cell lung cancer (NSCLC). Lung Cancer. 2010; 69(Suppl 1):S24–S30. PMID: 20727459.
Article3. Ando M, Saka H, Ando Y, Minami H, Kuzuya T, Yamamoto M, Watanabe A, Sakai S, Shimokata K, Hasegawa Y. Sequence effect of docetaxel and carboplatin on toxicity, tumor response and pharmacokinetics in non-small-cell lung cancer patients: a phase I study of two sequences. Cancer Chemother Pharmacol. 2005; 55:552–558. PMID: 15856233.
Article4. Lunardi G, Venturini M, Vannozzi MO, Tolino G, Del ML, Bighin C, Schettini G, Esposito M. Influence of alternate sequences of epirubicin and docetaxel on the pharmacokinetic behaviour of both drugs in advanced breast cancer. Ann Oncol. 2002; 13:280–285. PMID: 11886006.
Article5. Airoldi M, Cattel L, Marchionatti S, Recalenda V, Pedani F, Tagini V, Bumma C, Beatrice F, Succo G, Maria Gabriele A. Docetaxel and vinorelbine in recurrent head and neck cancer: pharmacokinetic and clinical results. Am J Clin Oncol. 2003; 26:378–381. PMID: 12902890.6. Dumez H, Louwerens M, Pawinsky A, Planting AS, de Jonge MJ, Van Oosterom AT, Highley M, Guetens G, Mantel M, de Boeck G, de Bruijn E, Verweij J. The impact of drug administration sequence and pharmacokinetic interaction in a phase I study of the combination of docetaxel and gemcitabine in patients with advanced solid tumors. Anticancer Drugs. 2002; 13:583–593. PMID: 12172503.
Article7. Schwantes U, Topfmeier P. Importance of pharmacological and physicochemical properties for tolerance of antimuscarinic drugs in the treatment of detrusor instability and detrusor hyperreflexia--chances for improvement of therapy. Int J Clin Pharmacol Ther. 1999; 37:209–218. PMID: 10363619.8. Kwon Y. Mechanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther. 2016; 9:2007–2016. PMID: 27103826.
Article9. Rosing H, Lustig V, van Warmerdam LJ, Huizing MT, ten Bokkel Huinink WW, Schellens JH, Rodenhuis S, Bult A, Beijnen JH. Pharmacokinetics and metabolism of docetaxel administered as a 1-h intravenous infusion. Cancer Chemother Pharmacol. 2000; 45:213–218. PMID: 10663639.
Article10. Bruno R, Riva A, Hille D, Lebecq A, Thomas L. Pharmacokinetic and pharmacodynamic properties of docetaxel: results of phase I and phase II trials. Am J Health Syst Pharm. 1997; 54(24 Suppl 2):S16–S19. PMID: 9435928.
Article11. Extra JM, Rousseau F, Bruno R, Clavel M, Le Bail N, Marty M. Phase I and pharmacokinetic study of Taxotere (RP 56976; NSC 628503) given as a short intravenous infusion. Cancer Res. 1993; 53:1037–1042. PMID: 8094996.12. Marre F, Sanderink GJ, de Sousa G, Gaillard C, Martinet M, Rahmani R. Hepatic biotransformation of docetaxel (Taxotere) in vitro: involvement of the CYP3A subfamily in humans. Cancer Res. 1996; 56:1296–1302. PMID: 8640817.13. Britten CD, Baker SD, Denis LJ, Johnson T, Drengler R, Siu LL, Duchin K, Kuhn J, Rowinsky EK. Oral paclitaxel and concurrent cyclosporin A: targeting clinically relevant systemic exposure to paclitaxel. Clin Cancer Res. 2000; 6:3459–3468. PMID: 10999729.14. Hirth J, Watkins PB, Strawderman M, Schott A, Bruno R, Baker LH. The effect of an individual's cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res. 2000; 6:1255–1258. PMID: 10778948.15. Gabizon AA, Shmeeda H, Zalipsky S. Pros and cons of the liposome platform in cancer drug targeting. J Liposome Res. 2006; 16:175–183. PMID: 16952872.
Article16. Harashima H, Tsuchihashi M, Iida S, Doi H, Kiwada H. Pharmacokinetic/pharmacodynamic modeling of antitumor agents encapsulated into liposomes. Adv Drug Deliv Rev. 1999; 40:39–61. PMID: 10837779.
Article17. Zamboni WC. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 2005; 11:8230–8234. PMID: 16322279.
Article18. von Tempelhoff GF, Heilmann L, Hommel G, Schneider D, Niemann F, Zoller H. Hyperviscosity syndrome in patients with ovarian carcinoma. Cancer. 1998; 82:1104–1111. PMID: 9506356.
Article19. Zhao L, Wei Y, Li W, Liu Y, Wang Y, Zhong X, Yu Y. Solid dispersion and effervescent techniques used to prepare docetaxel liposomes for lung-targeted delivery system: in vitro and in vivo evaluation. J Drug Target. 2011; 19:171–178. PMID: 20429774.20. Wang J, Lan Z, Zhang L, Guo H, Liu Z, Yu Y. A Rapid and sensitive UPLC-MS/MS method for determination of docetaxel in rabbit plasma: pharmacokinetic study of new lung-targeting docetaxel liposome at low dose. Cell Biochem Biophys. 2015; 73:623–629. PMID: 27259303.
Article21. Hooker AC, Ten Tije AJ, Carducci MA, Weber J, Garrett-Mayer E, Gelderblom H, McGuire WP, Verweij J, Karlsson MO, Baker SD. Population pharmacokinetic model for docetaxel in patients with varying degrees of liver function: incorporating cytochrome P4503A activity measurements. Clin Pharmacol Ther. 2008; 84:111–118. PMID: 18183036.22. Zhao L, Wei YM, Zhong XD, Liang Y, Zhang XM, Li W, Li BB, Wang Y, Yu Y. PK and tissue distribution of docetaxel in rabbits after i.v. administration of liposomal and injectable formulations. J Pharm Biomed Anal. 2009; 49:989–996. PMID: 19232851.
Article23. Knöspel F, Jacobs F, Freyer N, Damm G, De Bondt A, van den Wyngaert I, Snoeys J, Monshouwer M, Richter M, Strahl N, Seehofer D, Zeilinger K. In vitro model for hepatotoxicity studies based on primary human hepatocyte cultivation in a perfused 3D bioreactor system. Int J Mol Sci. 2016; 17:584. PMID: 27092500.
Article24. Bardelmeijer HA, Roelofs AB, Hillebrand MJ, Beijnen JH, Schellens JH, van Tellingen O. Metabolism of docetaxel in mice. Cancer Chemother Pharmacol. 2005; 56:299–306. PMID: 15864592.
Article25. Marlard M, Gaillard C, Sanderink GJ, Roberts S, Joannou P, Faccini V, Chapelle Ph, Freydman A. Kinetics, distribution, metabolism and excretion of radiolabelled Taxotere (14C-RP56976) in mice and dogs. Proc Am Assoc Cancer Res. 1993; 34:393.26. Vaclavikova R, Soucek P, Svobodova L, Anzenbacher P, Simek P, Guengerich FP, Gut I. Different in vitro metabolism of paclitaxel and docetaxel in humans, rats, pigs, and minipigs. Drug Metab Dispos. 2004; 32:666–674. PMID: 15155559.
Article27. Gaillard C, Monsarrat B, Vuilhorgne M, Royer I, Monegier B, Sable S, Guenard D, Gires P, Archimbaud Y, Wright M, Sanderink GJ. Docetaxel (Taxotere) metabolism in the rat in vivo and in vitro. Proc Am Assoc Cancer Res. 1994; 35:428.28. Monegier B, Gaillarda C, Sablé S, Vuilhorgne M. Structures of the major human metabolites of docetaxel (RP 56976 - Taxotere®). Tetrahedron Lett. 1994; 35:3715.
Article29. Sparreboom A, Van Tellingen O, Scherrenburg EJ, Boesen JJ, Huizing MT, Nooijen WJ, Versluis C, Beijnen JH. Isolation, purification and biological activity of major docetaxel metabolites from human feces. Drug Metab Dispos. 1996; 24:655–658. PMID: 8781781.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Concentration of Tobramycin in Cornea and Sclera After Subconjunctival Injection of Liposome-encapsulated Tobramycin in Rabbits
- The Effect of the Toxic Reaction of the Retina by Liposome-encapsulated Tobramycin in Normal Rabbits
- Pharmacokinetics of intravitreally injected liposome-encapsulated tobramycin in normal rabbits
- Effect of Bile Acids on Biliary Excretion of Cholesterol in Rabbits
- Effect of Main Pulmonary Artery Constriction on the Right Ventricle in Rabbits