Korean J Physiol Pharmacol.
2020 Jul;24(4):311-317. 10.4196/kjpp.2020.24.4.311.
Exploring the beneficial role of telmisartan in sepsis-induced myocardial injury through inhibition of high-mobility group box 1 and glycogen synthase kinase-3β/nuclear factor-κB pathway
- Affiliations
-
- 1Emergency Department, Second Affiliated Hospital of Dalian Medical University Dalian, Jinan, Shandong 116027, China
- 2Department of Cardiology, Hospital Affiliated to Shenyang Medical College, Shenyang, Liaoning 110021, P.R. China
- 3Innoscience Research Sdn Bhd, Subang Jaya, Selangor 47650, Malaysia
- 4Emergency Department, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong 250014, P.R. China
- KMID: 2503324
- DOI: http://doi.org/10.4196/kjpp.2020.24.4.311
Abstract
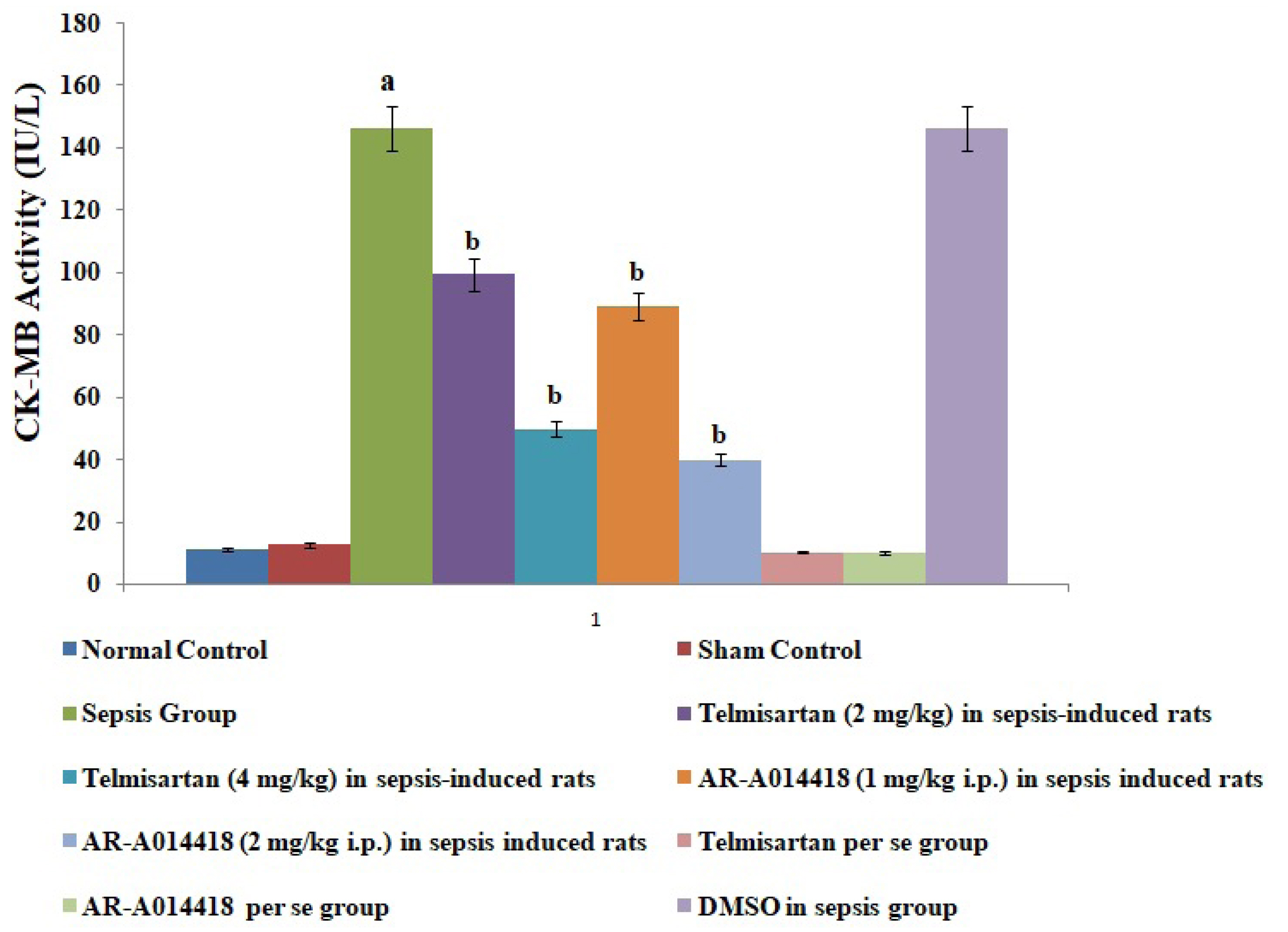
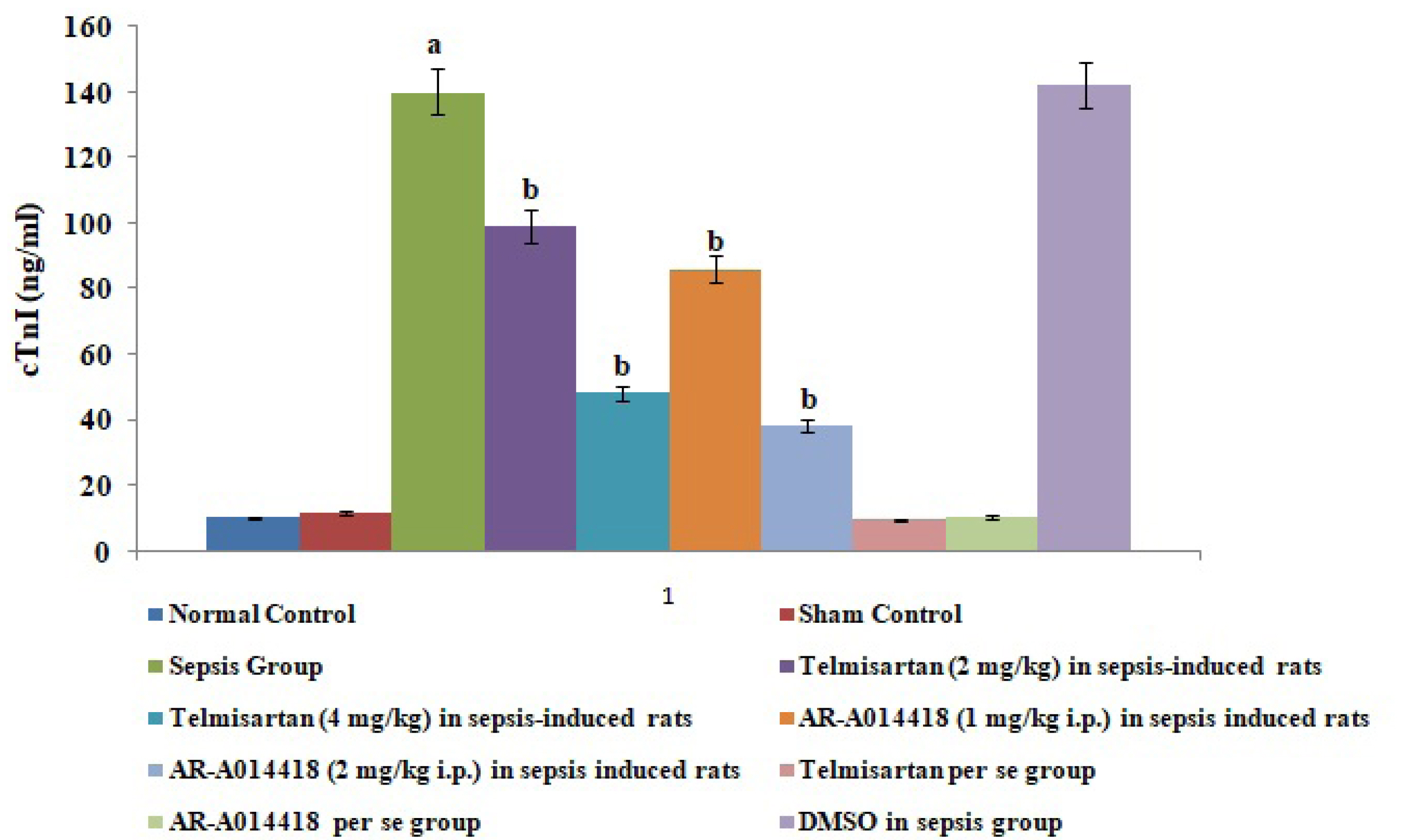
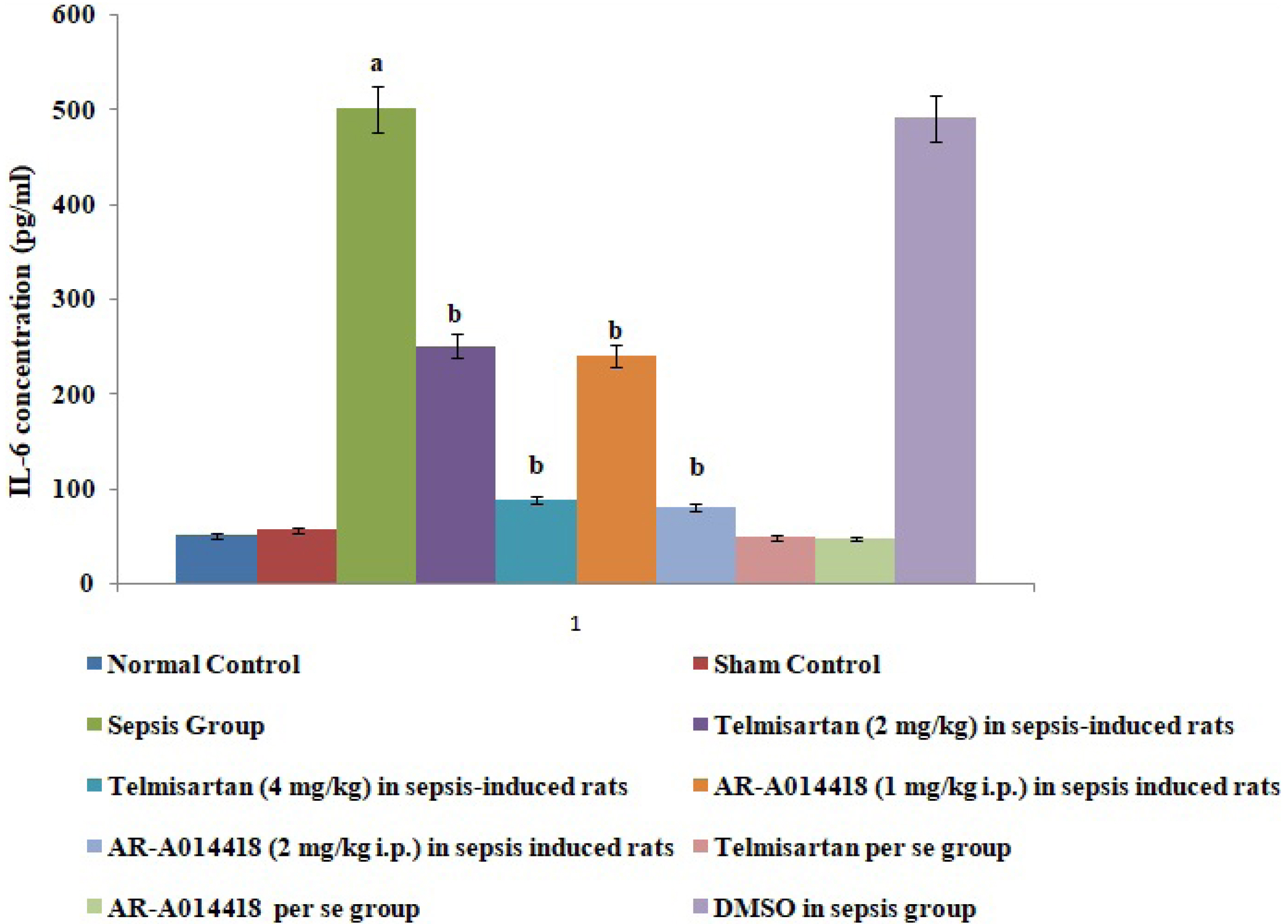
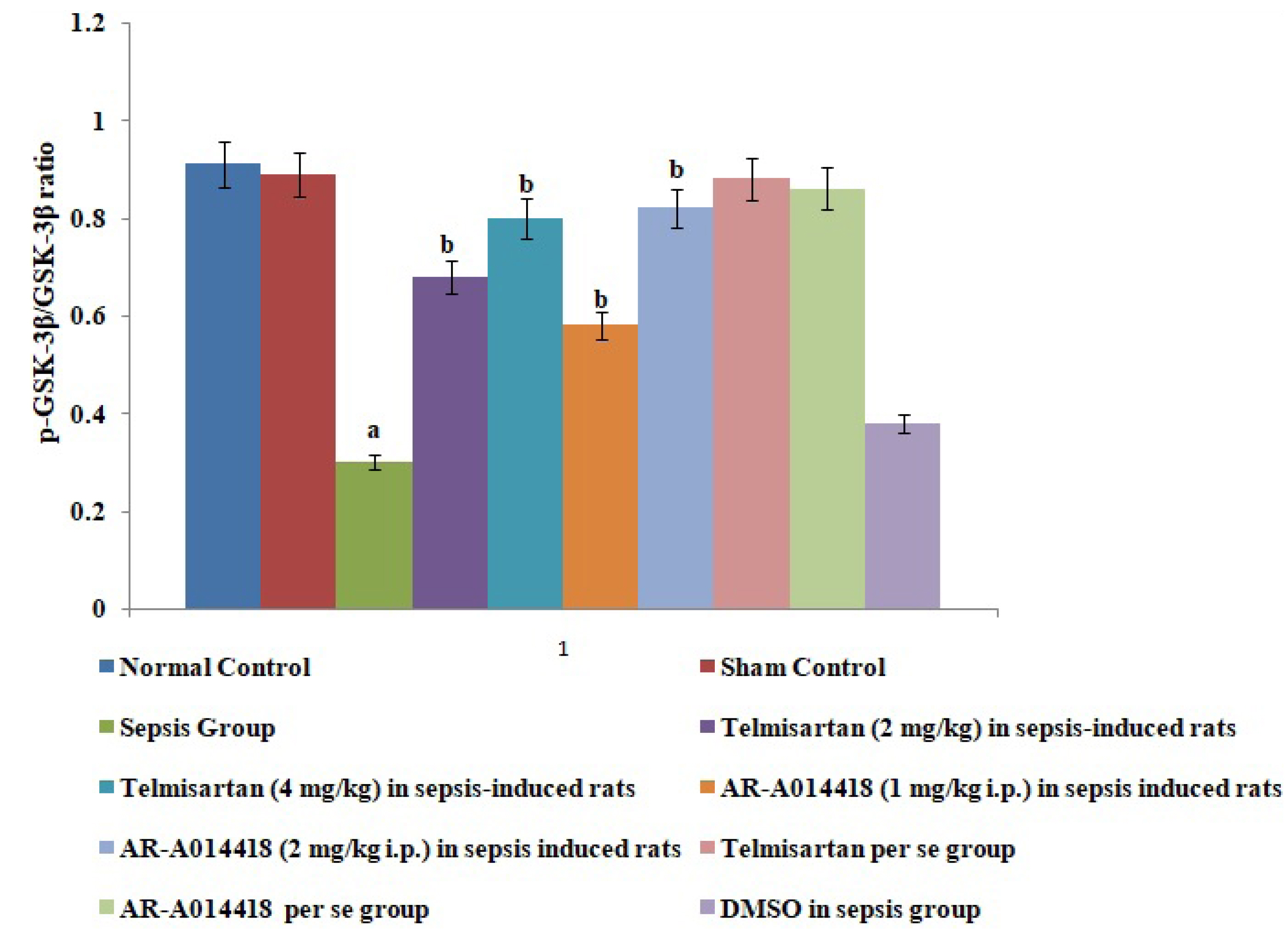
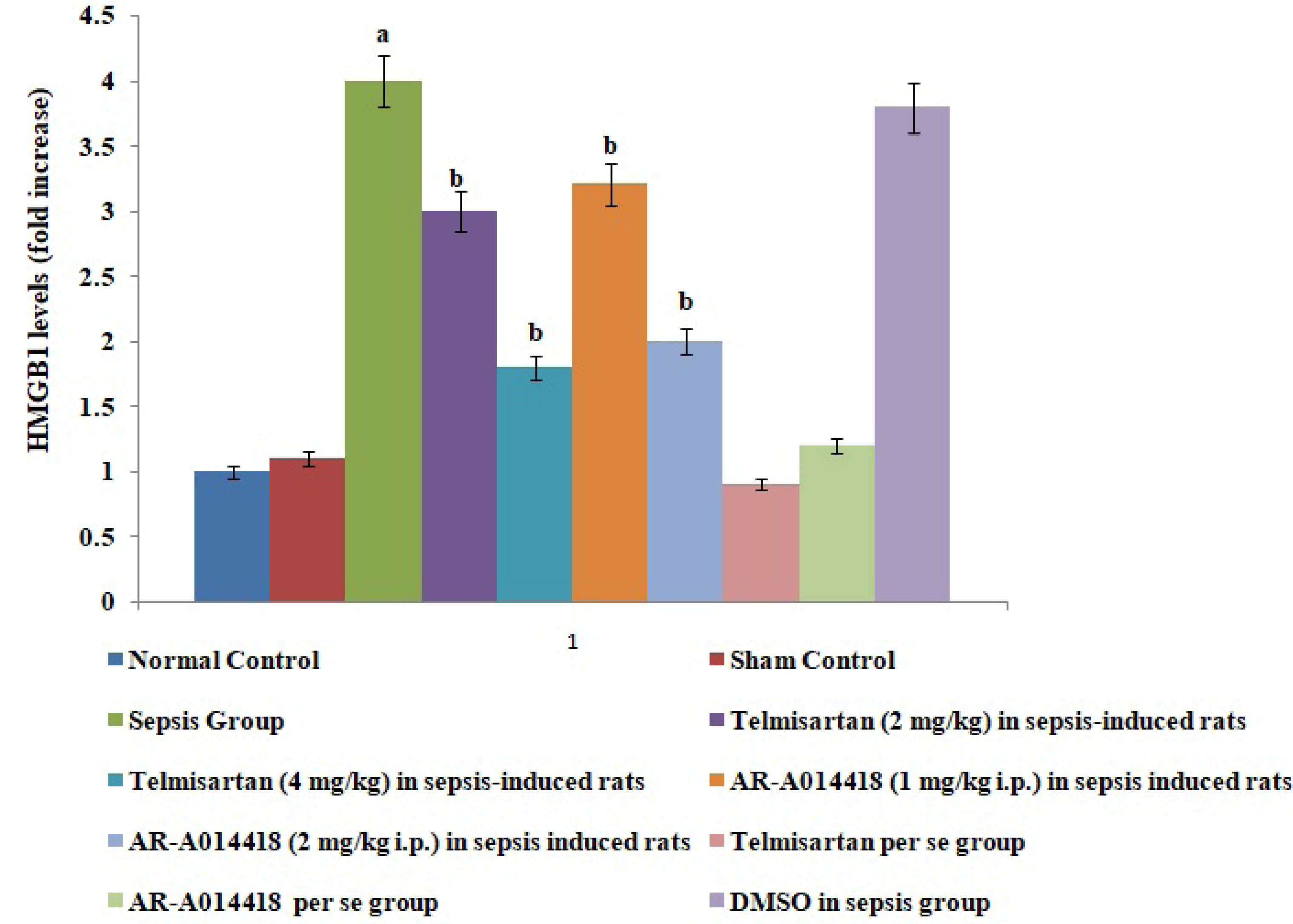
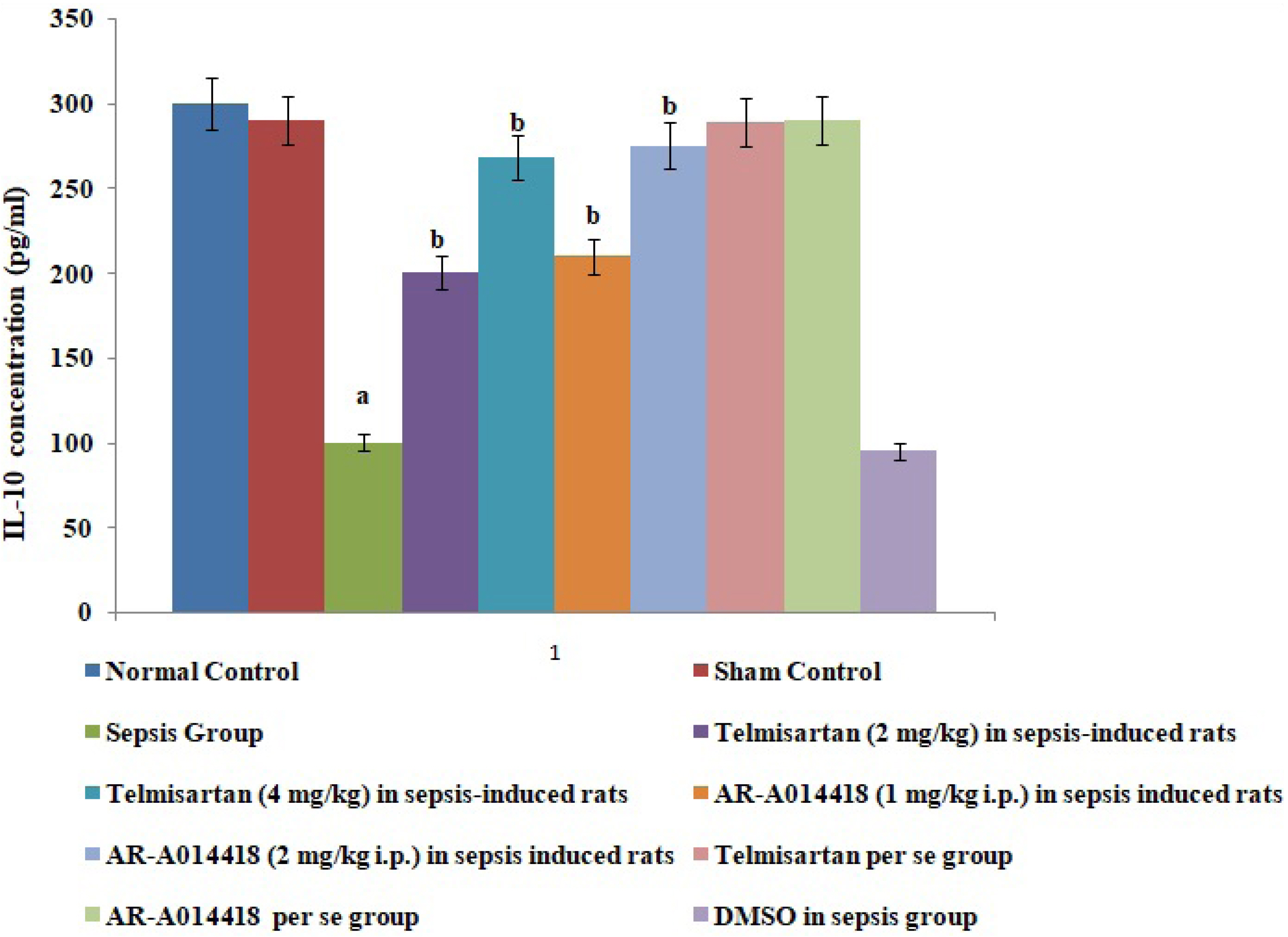
- In the present experimental study, cecal ligation and puncture significantly increased the myocardial injury assessed in terms of excess release of creative kinase-MB (CK-MB), cardiac troponin I (cTnI), interleukin (IL)-6 and decrease of IL-10 in the blood following 12 h of laparotomy procedure as compared to normal control. Also, a significant increase in protein expression levels of high-mobility group box 1 (HMGB1) and decreased phosphorylation of glycogen synthase kinase-3β (GSK-3β) was observed in the myocardial tissue as compared to normal control. A single independent administration of telmisartan (2 and 4 mg/kg) and AR-A014418 (1 and 2 mg/kg) substantially reduced sepsis-induced myocardial injury in terms of decrease levels of CK-MB, cTnI and IL-6, HMGB1, GSK-3β and increase in IL-10 and p-GSK-3β in the blood in sepsis- subjected rats. The effects of telmisartan at dose 4 mg/kg and AR-A014418 at a dose of 2 mg/kg were significantly higher than the telmisartan at a dose of 2 mg/kg and AR-A014418 1 mg/kg respectively. Further, no significant effects on different parameters were observed in the sham control group in comparison to normal. Therefore it is plausible to suggest that sepsis may increase the levels of angiotensin II to trigger GSK-3β-dependent signaling to activate the HMGB1/receptors for advanced glycation end products, which may promote inflammation and myocardial injury in sepsis-subjected rats.
Keyword
Figure
Cited by 1 articles
-
Fibroblast-derived interleukin-6 exacerbates adverse cardiac remodeling after myocardial infarction
Hongkun Li, Yunfei Bian
Korean J Physiol Pharmacol. 2024;28(3):285-294. doi: 10.4196/kjpp.2024.28.3.285.
Reference
-
1. Sagy M, Al-Qaqaa Y, Kim P. 2013; Definitions and pathophysiology of sepsis. Curr Probl Pediatr Adolesc Health Care. 43:260–263. DOI: 10.1016/j.cppeds.2013.10.001. PMID: 24295606.
Article2. Walley KR. 2018; Sepsis-induced myocardial dysfunction. Curr Opin Crit Care. 24:292–299. DOI: 10.1097/MCC.0000000000000507. PMID: 29846206.
Article3. Wang Z, Bu L, Yang P, Feng S, Xu F. 2019; Alleviation of sepsis induced cardiac dysfunction by overexpression of Sestrin2 is associated with inhibition of p S6K and activation of the p AMPK pathway. Mol Med Rep. 20:2511–2518. DOI: 10.3892/mmr.2019.10520. PMID: 31524263. PMCID: PMC6691248.4. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. 2001; Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 29:1303–1310. DOI: 10.1097/00003246-200107000-00002. PMID: 11445675.
Article5. Sifi A, Adi-Bessalem S, Laraba-Djebari F. 2017; Role of angiotensin II and angiotensin type-1 receptor in scorpion venom-induced cardiac and aortic tissue inflammation. Exp Mol Pathol. 102:32–40. DOI: 10.1016/j.yexmp.2016.11.006. PMID: 27955986.
Article6. Wang H, Ward MF, Sama AE. 2009; Novel HMGB1-inhibiting therapeutic agents for experimental sepsis. Shock. 32:348–357. DOI: 10.1097/SHK.0b013e3181a551bd. PMID: 19333143. PMCID: PMC2860725.
Article7. Tieu BC, Lee C, Sun H, Lejeune W, Recinos A 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. 2009; An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 119:3637–3651. DOI: 10.1172/JCI38308. PMID: 19920349. PMCID: PMC2786788.
Article8. Xu J, Carretero OA, Liao TD, Peng H, Shesely EG, Xu J, Liu TS, Yang JJ, Reudelhuber TL, Yang XP. 2010; Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 299:H1328–H1338. DOI: 10.1152/ajpheart.00538.2010. PMID: 20833959. PMCID: PMC2993216.
Article9. Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR. 2005; The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 201:1135–1143. DOI: 10.1084/jem.20042614. PMID: 15795240. PMCID: PMC2213120.
Article10. Bangert A, Andrassy M, Müller AM, Bockstahler M, Fischer A, Volz CH, Leib C, Göser S, Korkmaz-Icöz S, Zittrich S, Jungmann A, Lasitschka F, Pfitzer G, Müller OJ, Katus HA, Kaya Z. 2016; Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc Natl Acad Sci U S A. 113:E155–E164. DOI: 10.1073/pnas.1522288113. PMID: 26715748. PMCID: PMC4720305.
Article11. Volz HC, Kaya Z, Katus HA, Andrassy M. 2010; The role of HMGB1/RAGE in inflammatory cardiomyopathy. Semin Thromb Hemost. 36:185–194. DOI: 10.1055/s-0030-1251503. PMID: 20414834.
Article12. Wen H. 2013; Sepsis induced by cecal ligation and puncture. Methods Mol Biol. 1031:117–124. DOI: 10.1007/978-1-62703-481-4_15. PMID: 23824895. PMCID: PMC4081556.
Article13. Faix JD. 2013; Biomarkers of sepsis. Crit Rev Clin Lab Sci. 50:23–36. DOI: 10.3109/10408363.2013.764490. PMID: 23480440. PMCID: PMC3613962.
Article14. Takahashi-Yanaga F. 2018; Roles of glycogen synthase kinase-3 (GSK-3) in cardiac development and heart disease. J UOEH. 40:147–156. DOI: 10.7888/juoeh.40.147. PMID: 29925734.
Article15. Wei D, Xu H, Gai X, Jiang Y. 2019; Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3β signaling pathways. Acta Cir Bras. 34:e201900708. DOI: 10.1590/s0102-865020190070000008. PMID: 31531541. PMCID: PMC6746565.
Article16. Wong R, Aponte AM, Steenbergen C, Murphy E. 2010; Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol. 298:H75–H91. DOI: 10.1152/ajpheart.00515.2009. PMID: 19855063. PMCID: PMC2806145.
Article17. Wichterman KA, Baue AE, Chaudry IH. 1980; Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 29:189–201. DOI: 10.1016/0022-4804(80)90037-2.
Article18. Kikuchi K, Tancharoen S, Ito T, Morimoto-Yamashita Y, Miura N, Kawahara K, Maruyama I, Murai Y, Tanaka E. 2013; Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke. Int J Mol Sci. 14:18899–18924. DOI: 10.3390/ijms140918899. PMID: 24065095. PMCID: PMC3794813.
Article19. Nakamura K, Yamagishi S, Nakamura Y, Takenaka K, Matsui T, Jinnouchi Y, Imaizumi T. 2005; Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res. 70:137–141. DOI: 10.1016/j.mvr.2005.10.002. PMID: 16271939.
Article20. Zhang S, Qi W, Wang F, Xu Y, Wang X. 2019; [Effects of carbon monoxide release molecule-2 on sepsis-induced myocardial dysfunction in rats]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 31:1097–1101. Chinese. DOI: 10.3760/cma.j.issn.2095-4352.2019.09.008. PMID: 31657332.21. Lu ZQ, Lu JX, Deng YJ. 2015; Glucocorticoids offer protection against myocardial injury in a murine model of sepsis. Int J Clin Exp Med. 8:12211–12218.22. Sharma HS, Das DK. 1997; Role of cytokines in myocardial ischemia and reperfusion. Mediators Inflamm. 6:175–183. DOI: 10.1080/09629359791668. PMID: 18472818. PMCID: PMC2365828.
Article23. Li X, Cheng Q, Li J, He Y, Tian P, Xu C. 2017; Significance of hydrogen sulfide in sepsis-induced myocardial injury in rats. Exp Ther Med. 14:2153–2161. DOI: 10.3892/etm.2017.4742. PMID: 28962136. PMCID: PMC5609143.
Article24. O'Riordan CE, Purvis GSD, Collotta D, Chiazza F, Wissuwa B, Al Zoubi S, Stiehler L, Martin L, Coldewey SM, Collino M, Thiemermann C. 2019; Bruton's tyrosine kinase inhibition attenuates the cardiac dysfunction caused by cecal ligation and puncture in mice. Front Immunol. 10:2129. DOI: 10.3389/fimmu.2019.02129. PMID: 31552054. PMCID: PMC6743418.25. Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. 1999; HMG-1 as a late mediator of endotoxin lethality in mice. Science. 285:248–251. DOI: 10.1126/science.285.5425.248. PMID: 10398600.26. Zhen J, Li L, Yan J. 2018; [Advances in biomarkers of myocardial injury in sepsis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 30:699–702. Chinese. DOI: 10.3760/cma.j.issn.2095-4352.2018.07.017. PMID: 30045802.27. Xin Y, Bai Y, Jiang X, Zhou S, Wang Y, Wintergerst KA, Cui T, Ji H, Tan Y, Cai L. 2018; Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3b/Fyn pathway. Redox Biol. 15:405–417. DOI: 10.1016/j.redox.2017.12.016. PMID: 29353218. PMCID: PMC5975128.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Telmisartan Inhibits TNFα-Induced Leukocyte Adhesion by Blocking ICAM-1 Expression in Astroglial Cells but Not in Endothelial Cells
- Antineuroinflammatory Effects of 7,3’,4’-Trihydroxyisoflavone in Lipopolysaccharide-Stimulated BV2 Microglial Cells through MAPK and NF-κB Signaling Suppression
- Lithium alleviates paralysis in experimental autoimmune neuritis in Lewis rats by modulating glycogen synthase kinase-3β activity
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Neogambogic acid relieves myocardial injury induced by sepsis via p38 MAPK/NF-κB pathway