Korean J Physiol Pharmacol.
2022 Nov;26(6):511-518. 10.4196/kjpp.2022.26.6.511.
Neogambogic acid relieves myocardial injury induced by sepsis via p38 MAPK/NF-κB pathway
- Affiliations
-
- 1Department of Emergency, Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330000, China
- KMID: 2534526
- DOI: http://doi.org/10.4196/kjpp.2022.26.6.511
Abstract
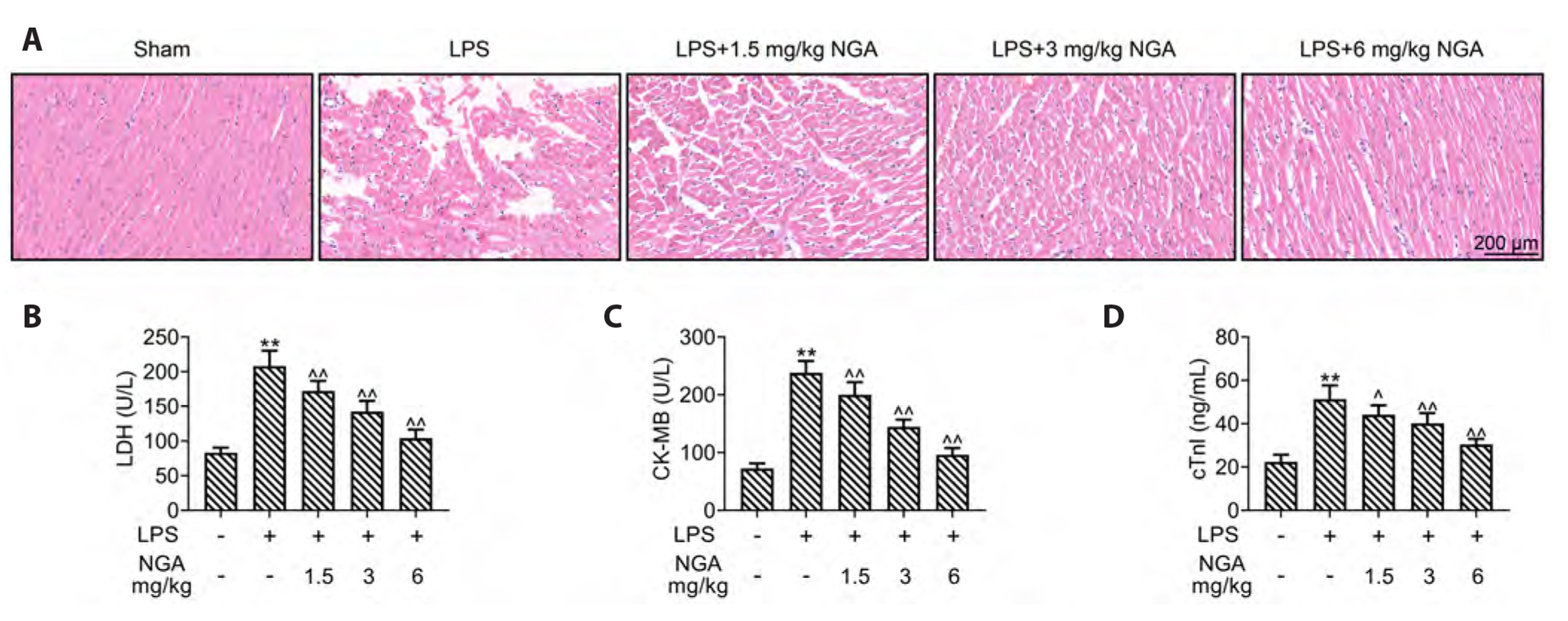
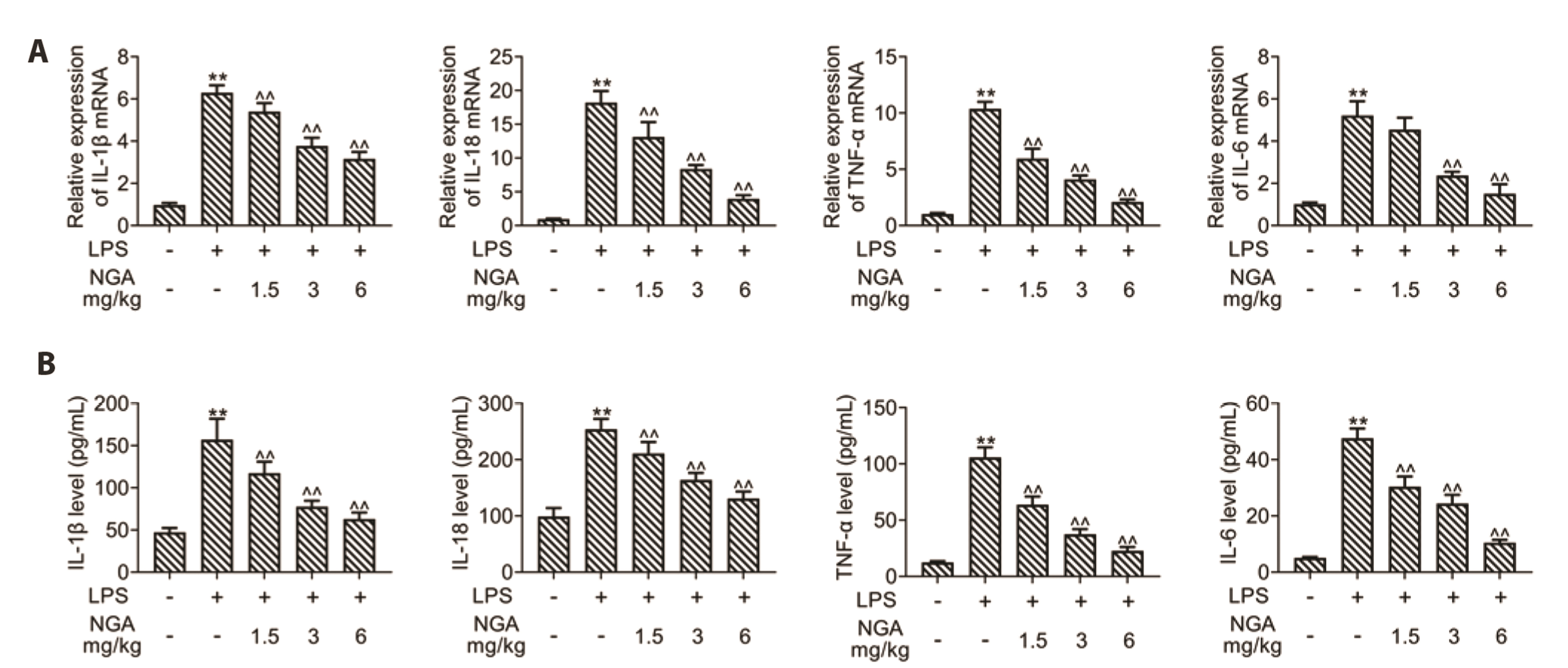
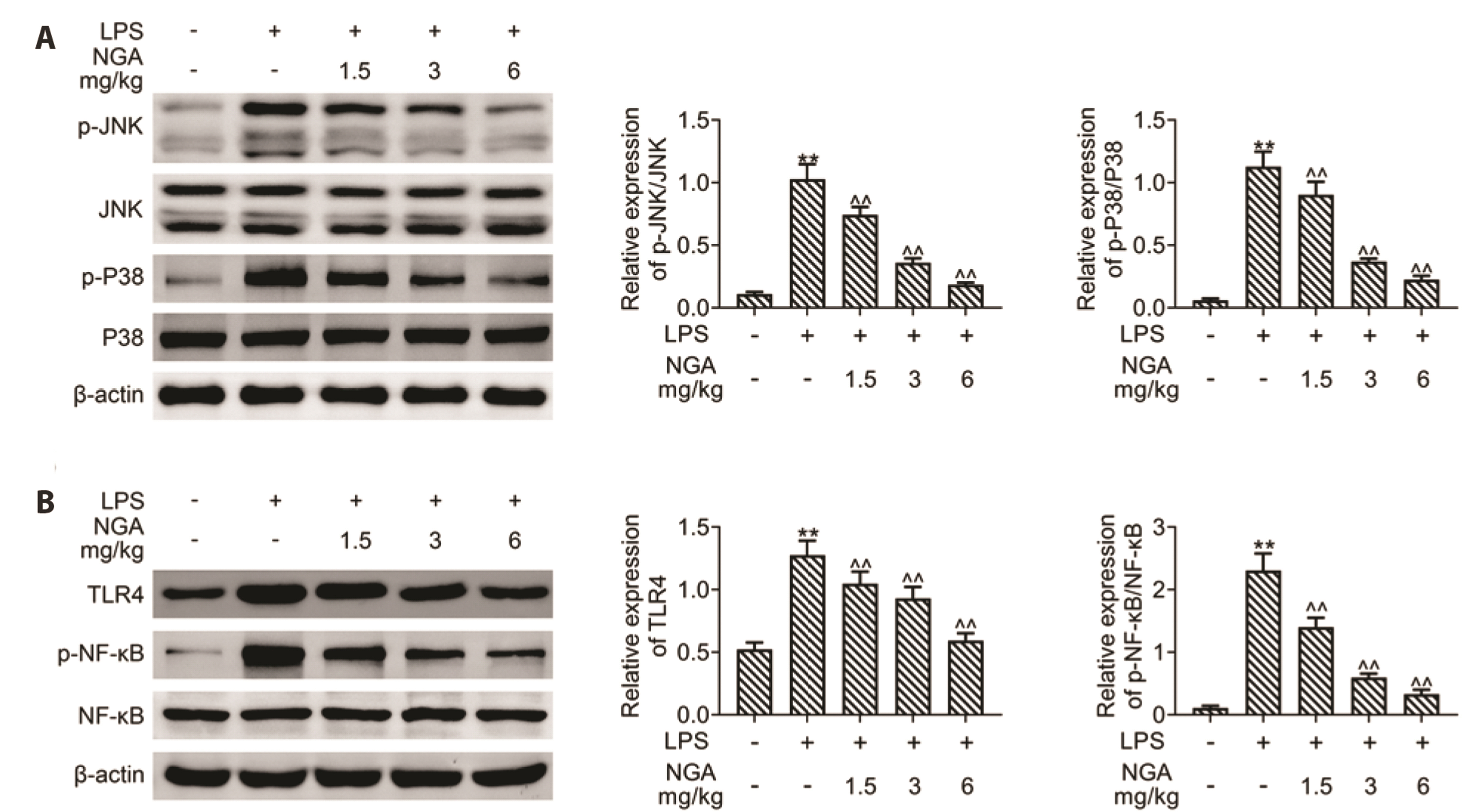
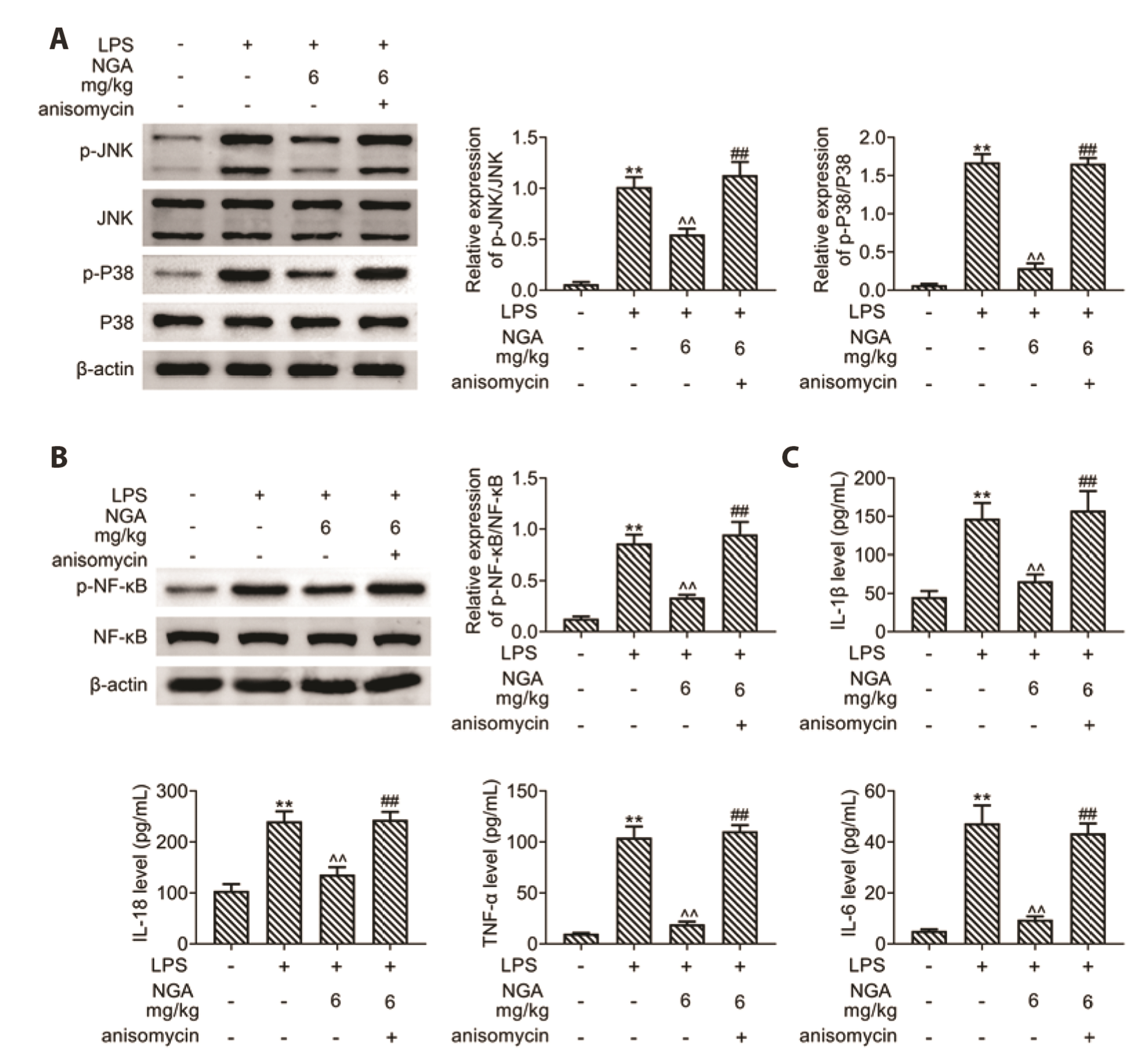
- Sepsis-associated myocardial injury, an invertible myocardial depression, is a common complication of sepsis. Neogambogic acid is an active compound in garcinia and exerts anthelmintic, anti-inflammatory, and detoxification properties. The role of neogambogic acid in sepsis-associated myocardial injury was assessed. Firstly, mice were pretreated with neogambogic acid and then subjected to lipopolysaccharide treatment to induce sepsis. Results showed that lipopolysaccharide treatment induced up-regulation of biomarkers involved in cardiac injury, including lactate dehydrogenase (LDH), creatine kinase-MB (CK-MB), and troponin I (cTnI). However, pretreatment with neogambogic acid reduced levels of LDH, CK-MB, and cTnI, and ameliorated histopathological changes in the heart tissues of septic mice. Secondly, neogambogic acid also improved cardiac function in septic mice through reduction in left ventricular end-diastolic pressure, and enhancement of ejection fraction, fractional shortening, and left ventricular systolic mean pressure. Moreover, neogambogic acid suppressed cardiac apoptosis and inflammation in septic mice and reduced cardiac fibrosis. Lastly, protein expression of p-p38, p-JNK, and p-NFκB in septic mice was decreased by neogambogic acid. In conclusion, neogambogic acid exerted anti-apoptotic, anti-fibrotic, and anti-inflammatory effects in septic mice through the inactivation of MAPK/NF-κB pathway.
Keyword
Figure
Cited by 1 articles
-
Fibroblast-derived interleukin-6 exacerbates adverse cardiac remodeling after myocardial infarction
Hongkun Li, Yunfei Bian
Korean J Physiol Pharmacol. 2024;28(3):285-294. doi: 10.4196/kjpp.2024.28.3.285.
Reference
-
1. Liang W, Guo L, Liu T, Qin S. 2021; MEF2C alleviates acute lung injury in cecal ligation and puncture (CLP)-induced sepsis rats by up-regulating AQP1. Allergol Immunopathol (Madr). 49:117–124. DOI: 10.15586/aei.v49i5.477. PMID: 34476932. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85115451426&origin=inward.
Article2. Song C, Adili A, Kari A, Abuduhaer A. 2021; FSTL1 aggravates sepsis-induced acute kidney injury through regulating TLR4/MyD88/NF-κB pathway in newborn rats. Signa Vitae. 17:167–173.3. L'Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG. 2020; Sepsis-induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep. 22:35. DOI: 10.1007/s11886-020-01277-2. PMID: 32377972. PMCID: PMC7222131. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084213564&origin=inward.4. Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. 2016; Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care. 4:22. DOI: 10.1186/s40560-016-0148-1. PMID: 27011791. PMCID: PMC4804632. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84991035135&origin=inward.
Article5. Ho J, Yu J, Wong SH, Zhang L, Liu X, Wong WT, Leung CC, Choi G, Wang MH, Gin T, Chan MT, Wu WK. 2016; Autophagy in sepsis: degradation into exhaustion? Autophagy. 12:1073–1082. DOI: 10.1080/15548627.2016.1179410. PMID: 27172163. PMCID: PMC4990998. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84979017547&origin=inward.
Article6. Sun Y, Yao X, Zhang QJ, Zhu M, Liu ZP, Ci B, Xie Y, Carlson D, Rothermel BA, Sun Y, Levine B, Hill JA, Wolf SE, Minei JP, Zang QS. 2018; Beclin-1-dependent autophagy protects the heart during sepsis. Circulation. 138:2247–2262. DOI: 10.1161/CIRCULATIONAHA.117.032821. PMID: 29853517. PMCID: PMC6274625. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056463995&origin=inward.
Article7. Zhao Q, Zhong J, Bi Y, Liu Y, Liu Y, Guo J, Pan L, Tan Y, Yu X. 2020; Gambogenic acid induces Noxa-mediated apoptosis in colorectal cancer through ROS-dependent activation of IRE1α/JNK. Phytomedicine. 78:153306. DOI: 10.1016/j.phymed.2020.153306. PMID: 32854039. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85089657488&origin=inward.
Article8. Yu X, Zhao Q, Zhang H, Fan C, Zhang X, Xie Q, Xu C, Liu Y, Wu X, Han Q, Zhang H. 2016; Gambogenic acid inhibits LPS-simulated inflammatory response by suppressing NF-κB and MAPK in macrophages. Acta Biochim Biophys Sin (Shanghai). 48:454–461. DOI: 10.1093/abbs/gmw021. PMID: 27025602. PMCID: PMC4888363. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84966534277&origin=inward.
Article9. Sun R, Zhang HM, Chen BA. 2018; Anticancer activity and underlying mechanism of neogambogic acid. Chin J Nat Med. 16:641–643. DOI: 10.1016/S1875-5364(18)30103-1. PMID: 30269840. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85054307696&origin=inward.
Article10. Hua X, Jia Y, Yang Q, Zhang W, Dong Z, Liu S. 2019; Transcriptional analysis of the effects of gambogic acid and neogambogic acid on methicillin-resistant Staphylococcus aureus. Front Pharmacol. 10:986. DOI: 10.3389/fphar.2019.00986. PMID: 31572177. PMCID: PMC6753875. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072935785&origin=inward.11. Zhang W, Zhang M, Wang Z, Cheng Y, Liu H, Zhou Z, Han B, Chen B, Yao H, Chao J. 2016; Neogambogic acid prevents silica-induced fibrosis via inhibition of high-mobility group box 1 and MCP-1-induced protein 1. Toxicol Appl Pharmacol. 309:129–140. DOI: 10.1016/j.taap.2016.09.003. PMID: 27616297. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84987622546&origin=inward.
Article12. Pandey MK, Karelia D, Amin SG. 2016; Gambogic acid and its role in chronic diseases. Adv Exp Med Biol. 928:375–395. DOI: 10.1007/978-3-319-41334-1_15. PMID: 27671824. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84988923217&origin=inward.
Article13. Lewis AJ, Seymour CW, Rosengart MR. 2016; Current murine models of sepsis. Surg Infect (Larchmt). 17:385–393. DOI: 10.1089/sur.2016.021. PMID: 27305321. PMCID: PMC4960474. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84979675873&origin=inward.
Article14. Wang S, Jia D, Lu H, Qu X. 2021; Paeoniflorin improves myocardial injury via p38 MAPK/NF-KB p65 inhibition in lipopolysaccharide-induced mouse. Ann Transl Med. 9:1449. DOI: 10.21037/atm-21-4049. PMID: 34734001. PMCID: PMC8506776.
Article15. Liu YC, Yu MM, Shou ST, Chai YF. 2017; Sepsis-induced cardiomyopathy: mechanisms and treatments. Front Immunol. 8:1021. DOI: 10.3389/fimmu.2017.01021. PMID: 28970829. PMCID: PMC5609588. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85028424501&origin=inward.
Article16. Eichenholz PW, Eichacker PQ, Hoffman WD, Banks SM, Parrillo JE, Danner RL, Natanson C. 1992; Tumor necrosis factor challenges in canines: patterns of cardiovascular dysfunction. Am J Physiol. 263(3 Pt 2):H668–H675. DOI: 10.1152/ajpheart.1992.263.3.H668. PMID: 1415590. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0026725685&origin=inward.
Article17. Pathan N, Franklin JL, Eleftherohorinou H, Wright VJ, Hemingway CA, Waddell SJ, Griffiths M, Dennis JL, Relman DA, Harding SE, Levin M. 2011; Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Crit Care Med. 39:1692–1711. DOI: 10.1097/CCM.0b013e3182186d27. PMID: 21494108. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79959661938&origin=inward.
Article18. Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, Tang Q. 2019; STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 24:101215. DOI: 10.1016/j.redox.2019.101215. PMID: 31121492. PMCID: PMC6529775. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85065764765&origin=inward.
Article19. Liu Q, Shan P, Li H. 2019; Gambogic acid prevents angiotensin II-induced abdominal aortic aneurysm through inflammatory and oxidative stress dependent targeting the PI3K/Akt/mTOR and NF-κB signaling pathways. Mol Med Rep. 19:1396–1402. DOI: 10.3892/mmr.2018.9720. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059504798&origin=inward.
Article20. Han CK, Tien YC, Jine-Yuan Hsieh D, Ho TJ, Lai CH, Yeh YL, Hsuan Day C, Shen CY, Hsu HH, Lin JY, Huang CY. 2017; Attenuation of the LPS-induced, ERK-mediated upregulation of fibrosis-related factors FGF-2, uPA, MMP-2, and MMP-9 by Carthamus tinctorius L in cardiomyoblasts. Environ Toxicol. 32:754–763. DOI: 10.1002/tox.22275. PMID: 27098997. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84964403973&origin=inward.
Article21. Huang GJ, Deng JS, Chen CC, Huang CJ, Sung PJ, Huang SS, Kuo YH. 2014; Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-κB and MAPK pathways in mice. J Agric Food Chem. 62:5321–5329. DOI: 10.1021/jf405113g. PMID: 24849405. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84902270954&origin=inward.
Article22. Jin G, Wang FF, Li T, Jia DD, Shen Y, Xu HC. 2018; Neogambogic acid suppresses receptor activator of nuclear factor κB ligand (RANKL)-induced osteoclastogenesis by inhibiting the JNK and NF-κB pathways in mouse bone marrow-derived monocyte/macrophages. Med Sci Monit. 24:2569–2577. DOI: 10.12659/MSM.909651. PMID: 29698379. PMCID: PMC5939603. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046692746&origin=inward.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kaempferol Regulates the Expression of Airway MUC5AC Mucin Gene via IκBα-NF-κB p65 and p38-p44/42-Sp1 Signaling Pathways
- D-Limonene mitigate myocardial injury in rats through MAPK/ ERK/NF-κB pathway inhibition
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- MIR210HG Aggravates Sepsis-Induced Inflammatory Response of Proximal Tubular Epithelial Cell via the NF-κB Signaling Pathway
- Blockade of p38 Mitogen-activated Protein Kinase Pathway Inhibits Interleukin-6 Release and Expression in Primary Neonatal Cardiomyocytes