Blood Res.
2020 Mar;55(1):35-43. 10.5045/br.2020.55.1.35.
Human platelet lysate efficiency, stability, and optimal heparin concentration required in culture of mammalian cells
- Affiliations
-
- 1Department of Biochemistry, Faculty of Pharmacy, Zagazig University, Egypt.
- 2National Organization for Research and Control of Biologicals, Giza, Egypt. akram.elhabab@yahoo.com
- KMID: 2472006
- DOI: http://doi.org/10.5045/br.2020.55.1.35
Abstract
- BACKGROUND
Fetal bovine serum (FBS) has been used to support the growth and proliferation of mammalian cells for decades. Owing to several risk factors associated with FBS, several trials have been conducted to evaluate substitutes to FBS with the same efficiency and the lower risk issues.
METHODS
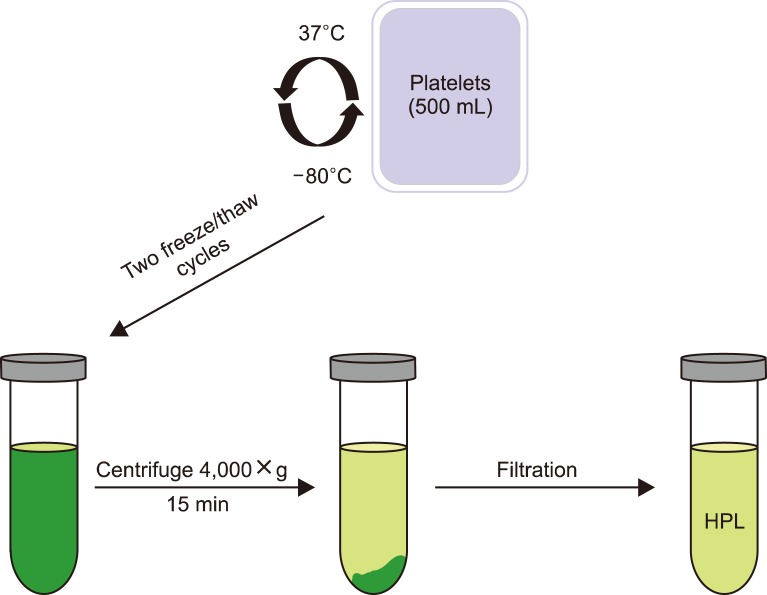
In this study, human platelet lysate (HPL) derived from activated human platelets was evaluated as an alternative to FBS due to the associated risk factors. To evaluate the efficiency of the preparation process, platelet count was performed before and after activation. The concentrations of several growth factors and proteins were measured to investigate HPL efficiency. HPL stability was studied at regular intervals, and optimal heparin concentration required to prevent gel formation in various media was determined. The biological activity of HPL and FBS was compared by evaluating the growth performance of Vero and Hep-2 cell lines.
RESULTS
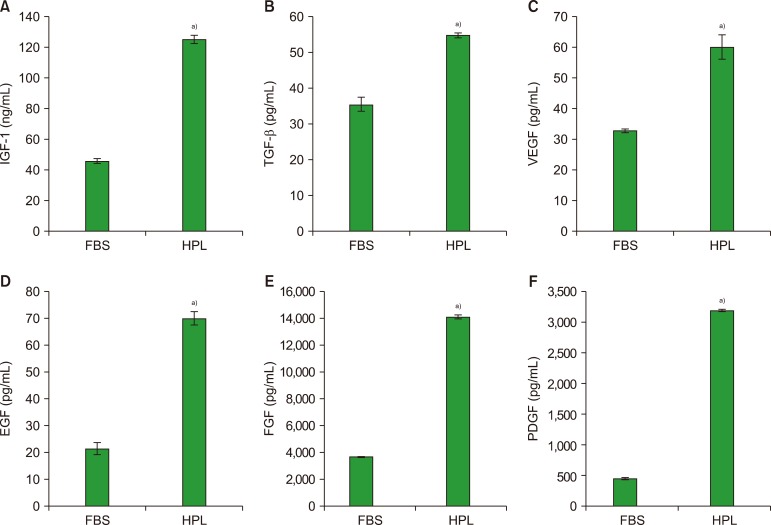
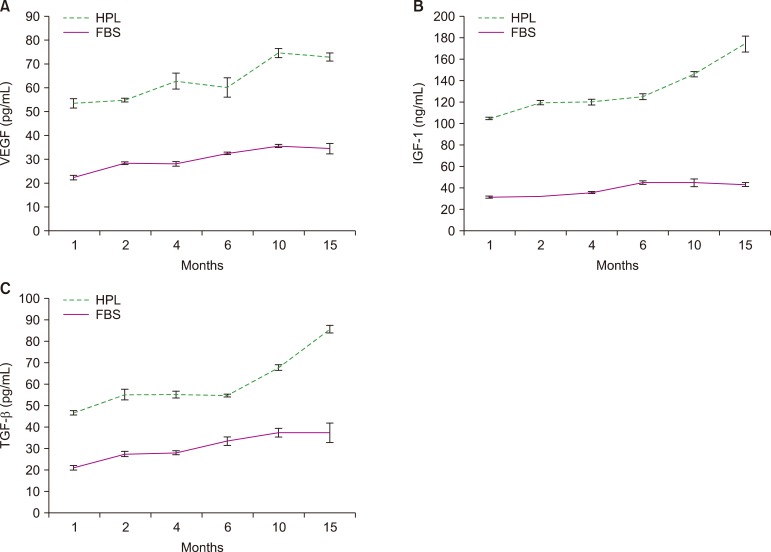
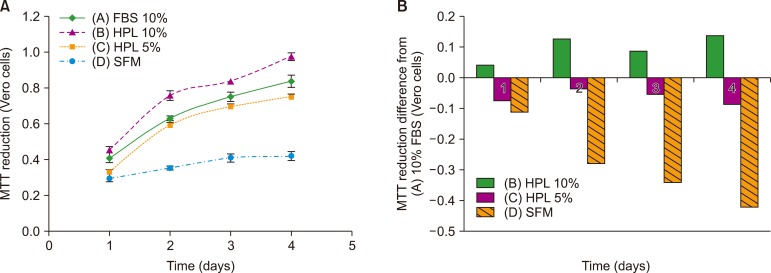
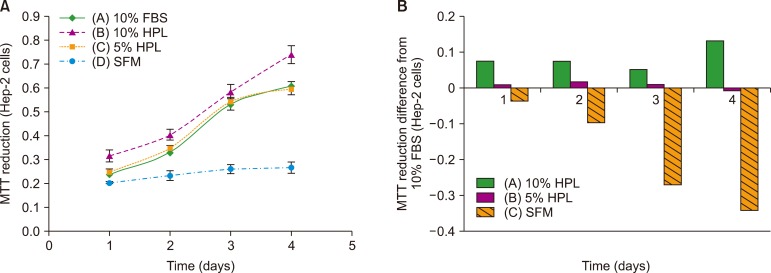
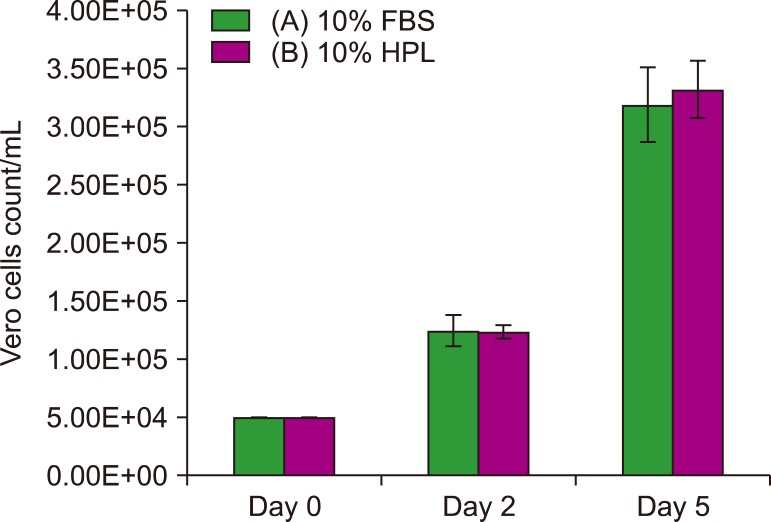
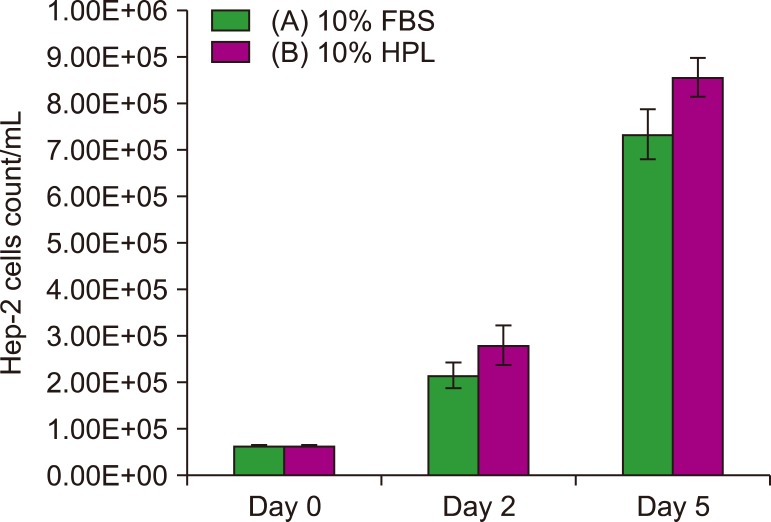
Result of platelet count assay revealed the efficiency of HPL preparation process. Growth factor concentrations in HPL were significantly higher than those in FBS, while the protein content of HPL was lower than that of FBS. Stability study data showed that the prepared HPL was stable for up to 15 months at −20℃. Ideal heparin concentration to be used in different media was dependent on calcium concentration. Results of cell viability assay showed that HPL was superior to FBS in supporting the growth and proliferation of Vero and Hep-2 cells.
CONCLUSION
The HPL prepared by the mechanical activation of platelets may serve as an efficient alternative to FBS in cell culture process.
Keyword
MeSH Terms
Figure
Reference
-
1. Butler M. Animal cell culture and technology. 2nd ed. Oxfordshire, UK: Taylor & Francis;2003.2. Jochems CE, van der Valk JB, Stafleu FR, Baumans V. The use of fetal bovine serum: ethical or scientific problem? Altern Lab Anim. 2002; 30:219–227. PMID: 11971757.
Article3. Montagnon BJ. Polio and rabies vaccines produced in continuous cell lines: a reality for Vero cell line. Dev Biol Stand. 1989; 70:27–47. PMID: 2759353.4. Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Expert Rev Vaccines. 2009; 8:607–618. PMID: 19397417.
Article5. Kenny MT, Schell K. Microassay of measles and mumps virus and antibody in VERO cells. J Biol Stand. 1975; 3:291–306. PMID: 1158903.
Article6. Yokomizo AY, Antoniazzi MM, Galdino PL, Azambuja N Jr, Jorge SA, Pereira CA. Rabies virus production in high vero cell density cultures on macroporous microcarriers. Biotechnol Bioeng. 2004; 85:506–515. PMID: 14760691.
Article7. Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. In : Martín J, editor. Poliovirus: methods and protocols. Totowa, NJ: Humana Press;2016. p. 145–176.8. Buchner C, Bryant C, Eslami A, Lakos G. Anti-nuclear antibody screening using HEp-2 cells. J Vis Exp. 2014; e51211. PMID: 24998977.
Article9. Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. 2014; 16:170–180. PMID: 24438898.
Article10. Burnouf T, Strunk D, Koh MB, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016; 76:371–387. PMID: 26561934.
Article11. Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E. Platelets and wound healing. Front Biosci. 2008; 13:3532–3548. PMID: 18508453.
Article12. Sittampalam GS, Brimacombe K, Arkin M, editors. Assay Guidance Manual. Bethesda, MD: Eli Lilly & Company and the National Center for Advancing Translational Sciences;2004.13. Louis KS, Siegel AC. Cell viability analysis using trypan blue: manual and automated methods. In : Stoddart MJ, editor. Mammalian cell viability: methods and protocols. Totowa, NJ: Humana Press;2011. p. 7–12.14. Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011; 105 Suppl 1:S13–S33. PMID: 21479340.
Article15. Allen AB, Butts EB, Copland IB, Stevens HY, Guldberg RE. Human platelet lysate supplementation of mesenchymal stromal cell delivery: issues of xenogenicity and species variability. J Tissue Eng Regen Med. 2017; 11:2876–2884. PMID: 27339032.
Article16. Roussy Y, Bertrand Duchesne MP, Gagnon G. Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res. 2007; 18:639–648. PMID: 17590158.
Article17. Baik SY, Lim YA, Kang SJ, Ahn SH, Lee WG, Kim CH. Effects of platelet lysate preparations on the proliferation of HaCaT cells. Ann Lab Med. 2014; 34:43–50. PMID: 24422195.
Article18. Schallmoser K, Strunk D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol Biol. 2013; 946:349–362. PMID: 23179843.
Article19. Pierce J, Benedetti E, Preslar A, et al. Comparative analyses of industrial-scale human platelet lysate preparations. Transfusion. 2017; 57:2858–2869. PMID: 28990195.
Article20. Ren J, Ward D, Chen S, et al. Comparison of human bone marrow stromal cells cultured in human platelet growth factors and fetal bovine serum. J Transl Med. 2018; 16:65. PMID: 29540180.
Article21. Rauch C, Feifel E, Amann EM, et al. Alternatives to the use of fetal bovine serum: human platelet lysates as a serum substitute in cell culture media. ALTEX. 2011; 28:305–316. PMID: 22130485.
Article22. Laner-Plamberger S, Lener T, Schmid D, et al. Mechanical fibrinogen-depletion supports heparin-free mesenchymal stem cell propagation in human platelet lysate. J Transl Med. 2015; 13:354. PMID: 26554451.
Article23. Gurbuz HA, Durukan AB, Sevim H, Ergin E, Gurpinar A, Yorgancioglu C. Heparin toxicity in cell culture: a critical link in translation of basic science to clinical practice. Blood Coagul Fibrinolysis. 2013; 24:742–745. PMID: 24064901.24. Hemeda H, Kalz J, Walenda G, Lohmann M, Wagner W. Heparin concentration is critical for cell culture with human platelet lysate. Cytotherapy. 2013; 15:1174–1181. PMID: 23845186.
Article25. Conrad DR. Calcium in cell culture. Darmstadt, Germany: Merck;2019. Accessed September 23, 2019. at https://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/calcium.html.26. Mikaelsson ME. The role of calcium in coagulation and anticoagulation. In : Smit Sibinga C, Das PC, Mannucci PM, editors. Coagulation and blood transfusion. Boston, MA: Springer US;1991. p. 29–37.27. Wang TJ, Chen MS, Chou ML, Lin HC, Seghatchian J, Burnouf T. Comparison of three human platelet lysates used as supplements for in vitro expansion of corneal endothelium cells. Transfus Apher Sci. 2017; 56:769–773. PMID: 28939367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect Erythrocyte lysate on Intracellular Calcium Concentration and the Potassium Channel in Cerebral Vasular Smooth Muscle Cells
- The Effect of Serum Concentrations of the Culture Media on the in vitro Secretion of Free beta-subunit of Human Chorionic Gonadotropin by Ovarian Cancer Cells
- The Effects of Human Bone Marrow-Derived Mesenchymal Stem Cell Conditioned Media Produced with Fetal Bovine Serum or Human Platelet Lysate on Skin Rejuvenation Characteristics
- Trends in Clinical Application of Platelet-Derived Bioproducts
- Culture of the Human Glomerular Endothelial Cells